Abstract
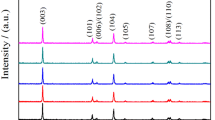
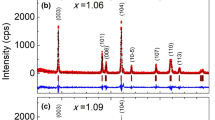
Layered structured LiNi0.5Mn0.5O2 and LiNi0.495M0.01Mn0.495O2 (M = Zn, Co, and Y) compounds were prepared by PVP (poly(vinyl pyrrolidone))-assisted sol-gel method, and their structural, morphological, vibrational, transport, and electrochemical properties were characterized by XRD, SEM, FTIR, Raman, AC impedance, and galvanostatic charge and discharge analysis. XRD patterns reveal that doping does not change the crystal structure of the LiNi0.5Mn0.5O2 compound. SEM images show that the average size of the particle is in sub-micron ranges. The AC impedance studies shows an electrical conductivity of ∼2.5 × 10−7 S/cm for the parent compound. The introduction of Zn/Co/Y at equivalent sites increased the electrical conductivity by one order ∼10−6 S/cm. The compound LiNi0.495Co0.01Mn0.495O2 shows the highest electrical conductivity of 2.85 × 10−6 S/cm and delivers a specific discharge capacity of 110 mAh/g at the end of the 25th cycle in the voltage window of 2.5–4.4 V for a current density of 30 mA/g.








Similar content being viewed by others
References
Mizushima K, Jones PC, Wiseman PJ, Goodenough JB (1980) LixCoO2 (0 < x < −1): a new cathode material for batteries of high energy density. Mater Res Bull 15:783–789
Hewston TA, Chamberland BL (1987) A survey of first row ternary oxides. J Phys Chem Solids 48:97
Myung ST, Komaba S, Hirosaki N, Hosaya K, Kumagai N (2005) Improvement of structural integrity and battery performance of LiNi0.5Mn0.5O2 by Al and Ti doping. J Power Sources 146:645–649
Nithya C, Lakshmi R, Gpoukumar S (2012) Effect of Mg dopant on the electrochemical performance of LiNi0.5Mn0.5O2 cathode materials for lithium rechargeable batteries. J Electrochem Soc 159:A1335–A1340
Ohzuku T, Makimura Y (2003) Lithium insertion material of LiNi1/2Mn1/2O2 for advanced lithium ion batteries. J Power Sources 119-121:156–160
Zhang B, Chen G, Xu P, Li CC (2008) Effect of equivalent and non-equivalent Al substitutions on the structure and electrochemical properties of LiNi0.5Mn0.5O2. J Power Sources 176:325–331
Li D, Sasaki Y, Kobayakawa K, Sato Y (2006) Impact of cobalt substitution for manganese on the structural and electrochemical properties of LiNi0.5Mn0.5O2. Electrochim Acta 51:3809–3813
Myung ST, Komaba S, Hirosaki N, Hosaya K, Miura Y, Kumagai N (2005) Synthesis of LiNi0.5Mn0.5-xTixO2 by an emulsion trying method and effect of Ti on structure and electrochemical properties. Chem Mater 17(9):2427–2435
Jeong KH, Ha HW, Yun NJ, Hong MZ, Kim K (2005) Zr doped Li[Ni0.5 − xMn0.5 − xZr2x]O2 (x = 0, 0.025) as cathode materials for lithium ion batteries. Electrochim Acta 50:5349–5353
Kim JH, Park CW, Sun YK (2003) Synthesis and electrochemical behavior of Li[Li0.1Ni0.35 – x / 2CoxMn0.35 – x / 2]O2 cathode materials. Solid State Ionics 164:43–39
Li D, Sasaki Y, Kobayakawa K, Sato Y (2006) Morphological, structural and electrochemical characteristics of LiNi0.5Mn0.4M0.1O2 (M = Li, Co, Mg, Al). J Power Sources 157:488–493
Senthil Kumar P, Sakunthala A, Prabu M (2014) Impact of cerium doping on the structural and electrical properties of lithium nickel manganese oxide (LiNi0.5Mn0.5O2). Int J Chem Tech Res 6:5252–5255
Senthil Kumar P, Sakunthala A, Prabu M, Reddy MV (2014) Transport properties of layered lithium nickel manganese oxide (LiNi0.5Mn0.5O2) cathode material for lithium ion battery. Proceedings of the 14th Asian Conference on Solid State Ionics, page: 13–22
Senthil Kumar P, Sakunthala A, Prabu M, Reddy MV, Joshi R (2014) Structure and electrical properties of lithium nickel manganese oxide (LiNi0.5Mn0.5O2) prepared by P123 assisted hydrothermal route. Solid State Ionics 267:1–8
Julien C (2000) Local cationic environment in lithium nickel–cobalt oxides used as cathode materials for lithium batteries. Solid State Ionics 887:136–137
Prabakaran SRS, Michael MS, Radhakrishnan S, Julien C (1997) Novel low temperature synthesis and characterization of LiNiVO4 for high voltage Li ion batteries. J Mater Chem 7:1791–1796
Sathiyamoorthi R, Shakkthivel P, Ramalakshmi S, Shul YG (2007) Influence of Mg doping on the performance of LiNiO2 matrix ceramic nanoparticles in high voltage lithium ion cells. J Power Sources 171:922–927
Julien C (2000) Local structure and electrochemistry of lithium cobalt oxides and their doped compounds. Solid State Ionics 157:57–71
Huang Y, Chen J, Ni J, Zhou H, Zhang X (2009) A modified ZrO2 coating process to improve electrochemical performance of LiNi1/3Co1/3Mn1/3O2. J Power Sources 188:538–545
Pant M, Kanchan DK, Gondaliya N (2009) Transport properties and relaxation studies in BaO substituted Ag2O-V2O5-TeO2 glass system. Mater Chem Phys 115:98–104
Prasad A, Basu A (2013) Dielectric and impedance properties of sintered magnesium aluminium silicate glass-ceramic. J Adv Ceram 2(1):71–78
Tewari S, Ghosh A, Bhattacharjee A (2016) Studies on frequency dependent electrical and dielectric properties of zinc oxide pellets: effect of Al doping. Indian J Phys. doi:10.1007/s12648-016-0858-1
Senthil Kumar P, Sakunthala A, Reddy MV, Shanmugam S, Prabu M (2016) Correlation between the structural, electrical and electrochemical performance of layered Li(Ni0.33Co0.33Mn0.33)O2 for lithium ion battery. J Solid State Electrochem 20:1865–1876
Yuan X, Xu QJ, Wang C, Liu X, Liu H, Xia Y (2016) A facile and novel organic co-precipitation strategy to prepare layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high capacity and excellent cycling stability. J Power Sources 279:157–164
Yuan X, Xu QJ, Liu X, Shen W, Liu H, Xia Y (2016) Excellent rate performance and high capacity of Mo doped layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 derived from an improved co-precipitation approach. Electrochim Acta 207:120–129
Liu X, Si W, Zhang J, Sun X, Deng J, Baunack S, Oswald S, Liu L, Yan C, Schmidt OG (2014) Free-standing Fe2O3 nano membranes enabling ultra-long cycling life and high rate capability for Li-ion batteries. Scientific Reports 4:7452. doi:10.1038/srep07452
Yuan X, Xu Q-j, Wang C, Liu X, Liu H, Xia Y (2015) A facile and novel organic coprecipitation strategy to prepare layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high capacity and excellent cycling stability. J Power Sources 279:157–164
Jin X, Xu Q, Liu H, Yuan X, Xia Y (2014) Excellent rate capability of Mg doped Li[Li0.2Ni0.13Co0.13Mn0.54]O2 cathode material for lithium-ion battery. Electrochim Acta 136:19–26
Yuan X, Xu Q-j, Liu X, Shen W, Liu H, Xia Y (2016) Excellent rate performance and high capacity of Mo doped layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 derived from an improved coprecipitation approach. Electrochim Acta 207:120–129
Acknowledgement
The authors would like to thank Karunya University, Coimbatore- 641114, Tamilnadu, India for the central research facilities. The authors are very much grateful to the Department of Atomic Energy, Board of Research in Nuclear Sciences, Mumbai (DAE-BRNS Project No. 34/32/1221/2012) for providing funding for this research work. One of the authors, Mr. P. Senthil Kumar, thanks DAE-BRNS (Project No. 34/32/1221/2012), for the grant of Senior Research Fellowship. The authors are also thankful to the Department of Science and Technology (DST), India (Project No. SR/FTP/PS-192/2011) for the financial support and SK thanks NUS IRI for providing research opportunities at NUS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthil Kumar, P., Sakunthala, A., Reddy, M.V. et al. Preparation and characterization of LiNi0.495M0.01Mn0.495O2 (M = Zn, Co, and Y) for lithium ion batteries. Ionics 23, 3013–3022 (2017). https://doi.org/10.1007/s11581-017-2110-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2110-1