Abstract
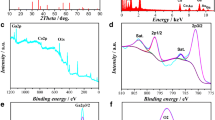
We report the studies on quasi-solid battery-supercapacitor (BatCap) systems fabricated using sol–gel-prepared LiFePO4 and its composites (LACs) with activated charcoal (AC) as hybrid cathode and Li4Ti5O12 powder as anode separator by flexible gel polymer electrolyte (GPE) film. The GPE film comprises 1.0 M lithium trifluoromethane sulfonate (LiTf) solution in ethylene carbonate (EC)–propylene carbonate (PC) mixture, immobilized poly(vinylidene fluoride-co-hexafluoro-propylene) (PVdF-HFP), which is of high ionic conductivity (∼3.8 × 10−3 S cm−1 at 25 °C) and electrochemical stability window (∼3 V). The effect of the addition of AC in composite electrode LACs has been analyzed using various techniques such as X-ray diffraction, porosity analysis, and electrochemical methods. The interfaces of composite LACs and GPE film not only offer high rate performance but also show high specific energy (>27.8 Wh kg−1) as compared to the symmetric supercapacitors and pristine lithium iron phosphate (LiFePO4)-based lithium ion batteries. The full BatCap systems have been characterized by cyclic voltammetry and galvanostatic charge–discharge tests. The BatCap systems with composite electrodes (LACs) offer better cyclic performance as compared to that of pristine LiFePO4-based BatCap or LIB LiFePO4/Li4Ti5O12.









Similar content being viewed by others
References
Beguin F, Frackowiak E (2013) Supercapacitors materials, systems, and applications. Wiley-VCH Verlag GmbH & Co, KGaA, Germany
Luo X, Wang J, Dooner M, Clarke J (2015) Overview of current development in electrical energy storage technologies and the application potential in power system operation. Appl Energy 137:511–536
Conway BE (1999) Electrochemical supercapacitors: scientific fundamentals and technological applications. Kluwer Academic/Plenum, New York
Hashmi SA (2004) Supercapacitor: an emerging power source. SciLett 27:27–46
Zhong C, Deng Y, Hu W, Qiao J, Zhang L, Zhang J (2015) A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem Soc Rev 44:7484–7539
Roldan S, Barreda D, Granda M, Menendez R, Santamarıa R, Blanco C (2015) An approach to classification and capacitance expressions in electrochemical capacitors technology. Phys Chem Chem Phys 17:1084–1092
Pell WG, Conway BE (2004) Peculiarities and requirements of asymmetric capacitor devices based on combination of capacitor and battery-type electrodes. J Power Sources 136:334–345
Amatucci GG, Badway F, Pasquier AD, Zheng T (2001) An asymmetric hybrid nonaqueous energy storage cell. J Electrochem Soc 148:A930–AA39
Cericola D, Kötz R (2012) Hybridization of rechargeable batteries and electrochemical capacitors: principles and limits. Electrochim Acta 72:1–17
Goodenough JB, Park KS (2013) The Li-ion rechargeable battery: a perspective. J Am ChemSoc 135:1167–1176
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195:939–954
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188–1194
Yuan LX, Wang ZH, Zhang WX, Hu XL, Chen JT, Huang YH, Goodenough JB (2011) Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy Environ Sci 4:269–284
Zhang WJ (2011) Structure and performance of LiFePO4 cathode materials: a review. J Power Sources 196:2962–2970
Chung SY, Bloking JT, Chiang YM (2002) Electronically conductive phospho-olivines as lithium storage electrodes. Nat Mater 1:123–128
Jin B, Jin EM, Park KH, Gu HB (2008) Electrochemical properties of LiFePO4-multiwalled carbon nanotubes composite cathode materials for lithium polymer battery. Electrochem Commun 10:1537–1540
Zhang H, Chen Y, Zheng C, Zhang D, He C (2015) Enhancement of the electrochemical performance of LiFePO4/carbon nanotubes composite electrode for Li-ion batteries. Ionics 21:1813–1818
Singh MK, Hashmi SA (2017) Performance studies on hybrid battery-supercapacitor fabricated with LiFePO4@MWCNTs nanocomposite cathode and gel polymer electrolyte. Ionics Communicated
Oh SM, Oh SW, Yoon CS, Scrosati B, Amine K, Sun YK (2010) High-performance carbon-LiMnPO4 nanocomposite cathode for lithium batteries. Adv Funct Mater 20:3260–3265
Böckenfeld N, Kühnel RS, Passerini S, Winter M, Balducci A (2011) Composite LiFePO4/AC high rate performance electrodes for Li-ion capacitors. J Power Sources 196:4136–4142
Wang G, Liu H, Liu J, Qiao S, Lu GM, Munroe P, Ahn H (2010) Mesoporous LiFePO4/C nanocomposite cathode materials for high power lithium ion batteries with superior performance. Adv Mater 22:4944–4948
Johns PA, Roberts MR, Wakizaka Y, Sanders JH, Owen JR (2009) How the electrolyte limits fast discharge in nanostructured batteries and supercapacitors. Electrochem Commun 11:2089–2092
Bockenfeld N, Placke T, Winter M, Passerini S, Balducci A (2012) The influence of activated carbon on the performance of lithium iron phosphate based electrodes. Electrochim Acta 76:130–136
Wang B, Wang Q, Xu B, Liu T, Wang D, Zhao G (2013) The synergy effect on Li storage of LiFePO4 with activated carbon modifications. RSC Adv 3:20024–20033
Hu XB, Huai YJ, Lin ZJ, Suo JS, Deng ZH (2007) A (LiFePO4-AC)/ Li4Ti5O12 hybrid battery capacitor. J Electrochem Soc 154:A1026–A1030
Goriparti S, Miele E, Angelis FD, Fabrizio ED, Zaccaria RP, Capiglia C (2014) Review on recent progress of nanostructured anode materials for Li-ion batteries. J Power Sources 257:421–443
Scrosati B, Garche J (2010) Lithium batteries: status, prospects and future. J Power Sources 195:2419–2430
Cui W, He YB, Tang ZY, Yang QH, Xu Q, Su FY, Ma L (2012) Improvement of overcharge performance using Li4Ti5O12 as negative electrode for LiFePO4 power battery. J Solid State Electrochem 16:265–271
Abe T (2009) Secondary batteries-lithium rechargeable systems lithium-ion/negative electrodes: carbon. Elsevier, Amsterdam
Zhao B, Ran R, Liu M, Shao Z (2015) A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: the latest advancements and future perspectives. Materials Science and Engineering: R: Reports 98:1–71
Yi TF, Jiang LJ, Shu J, Yue CB, Zhu RS, Qiao HB (2010) Recent development and application of Li4Ti5O12 as anode material of lithium ion battery. J Phys Chem Solids 71:1236–1242
Liu Y, Gorgutsa S, Santato C, Skorobogatiyz M (2012) The electrochemical society flexible, solid electrolyte-based lithium battery composed of LiFePO4 cathode and Li4Ti5O12 anode for applications in smart textiles. J Electrochem Soc 159(4):A349–A356
Lee SC, Lee SM, Lee JW, Lee JB, Lee SM, Han SS, Lee HC, Kim HJ (2009) Spinel Li4Ti5O12 nanotubes for energy storage materials. J Phys Chem C113:18420–18423
Agrawal RC, Pandey GP (2008) Solid polymer electrolytes: materials designing and all-solid-state battery applications: an overview. J Phys D Appl Phys 41:223001 (18pp)
Stephan AM, Nahm KS (2006) Review on composite polymer electrolytes for lithium batteries. Polymer 47:5952–5964
Singh MK, Kumar Y, Hashmi SA (2013) ‘Bucky gel’ of multiwalled carbon nanotubes as electrodes for high performance, flexible electric double layer capacitors. Nanotechnology 24:465704 (10pp)
Sivaraman P, Thakur A, Kushwaha RK, Ratna D, Samui AB (2006) Poly(3-methyl thiophene)-activated carbon hybrid supercapacitor based on gel polymer electrolyte. Electrochem Solid-State Lett 9(9):A435–A438
Sanchez M, Brito G, Fantini M, Goya G, Matos J (2006) Synthesis and characterization of LiFePO4 prepared by sol-gel technique. Solid State Ionics 177:497–500
Pandey GP, Hashmi SA, Kumar Y (2010) Performance studies of activated charcoal based electrical double layer capacitors with ionic liquid gel polymer electrolytes. Energy Fuel 24(12):6644–6652
Kumar D, Suleman M, Hashmi SA (2011) Studies on poly (vinylidene fluoride-co-hexafluoropropylene) based gel electrolyte nanocomposite for sodium-sulfur batteries. Solid State Ionics 202:45–53
Maccallum JR, Vincent CA (1987) Polymer electrolyte review—I. Elsevier Applied Science Publications Ltd, New York
Angell CA (1997) Why C = 16–17 in the WLF equation is physical and the fragility of polymers. Polymer 38:6261–6266
Hashmi SA, Bhat MY, Singh MK, Sundaram NTK, Raghupathy BPC, Tanaka H (2016) Ionic liquid-based sodium ion-conducting composite gel polymer electrolytes: effect of active and passive fillers. J Solid State Electrochem 20:2817–2826
Rouquerol KJ, Rouquerol F (1998) Adsorption by powders and porous solids: principles, methodology and applications. Academic Press, London
Hong SA, Kim SJ, Kim J, Lee BG, Chung KY, Lee YW (2012) Carbon coating on lithium iron phosphate (LiFePO4): comparison between continuous supercritical hydrothermal method and solid-state method. Chem Eng J 198:318–326
Bai Y, Yin Y, Yang J, Qing C, Zhang W (2011) Raman study of pure, C-coated and Co-doped LiFePO4: thermal effect and phase stability upon laser heating. J Raman Spectrosc 42:831–838
Shang H, Lu Y, Zhao F, Chao C, Zhang B, Zhang H (2015) Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv 5:75728–75734
Singh MK, Suleman M, Kumar Y, Hashmi SA (2015) A novel configuration of electrical double layer capacitor with plastic crystal based gel polymer electrolyte and graphene nano-platelets as electrodes: a high rate performance. Energy 80:465–473
Wang W, Daiwon C, Zhenguo Y (2013) Li-ion battery with LiFePO4 cathode and Li4Ti5O12 anode for stationary energy storage. Metallurgical and Materials Transactions 44A:S21–S25
Pasquier AD, Plitz I, Gural J, Menocal S, Amatucci G (2003) Characteristics and performance of 500 F asymmetric hybrid advanced supercapacitor prototypes. J Power Sources 113:62–71
Wang YG, Luo JY, Wang CX, Xia YY (2006) Hybrid aqueous energy storage cells using activated carbon and lithium ion intercalated compounds II. Comparison of LiMn2O4, LiCo1/3Ni1/3Mn1/3O2, and LiCoO2 positive electrodes. J Electrochem Soc 153:A1425–A1431
Wu H, Rao CV, Rambabu B (2009) Electrochemical performance of LiNi0.5Mn1.5O4 prepared by improved solid state method as cathode in hybrid supercapacitor. Mater Chem Phys 116:532–535
Wang YG, Xia YY (2005) A new concept hybrid electrochemical surpercapacitor: carbon/LiMn2O4 aqueous system. Electrochem Commun 7(11):1138–1142
Vasanthi R, Kalpana D, Renganathan NG (2008) Olivine-type nanoparticle for hybrid supercapacitors. J Solid State Electrochem 12:961–969
Ling P, JiaMing Z, ZhiQiang S, Jie Q, Wang CY (2012) Electrochemical performance of MCMB/(AC+LiFePO4) lithium-ion capacitors. Chin Sci Bull 58(6):689–695
Hu X, Deng Z, Suo J, Pan Z (2009) A high rate, high capacity and long life (LiMn2O4+ AC)/Li4Ti5O12 hybrid battery-supercapacitor. J Power Sources 187(2):635–639
Hu XB, Lin ZJ, Liu L, Huai YJ, Deng ZH (2010) Effects of the LiFePO4 content and he preparation method on the properties of (LiFePO4+ AC)/Li4Ti5O12 hybrid battery-capacitors. Journal of the serbian chemistry society 75(9):1259–1269
Chen S, Hu H, Wang C, Wang G, Yin J, Cao D (2012) (LiFePO4-AC)/Li4Ti5O12 hybrid supercapacitor: the effect of LiFePO4 content on its performance. J Renewable Sustainable Energy 4:033114 (1-4)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, M.K., Hashmi, S.A. Performance of solid-state hybrid supercapacitor with LiFePO4/AC composite cathode and Li4Ti5O12 as anode. Ionics 23, 2931–2942 (2017). https://doi.org/10.1007/s11581-017-2027-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-017-2027-8