Abstract
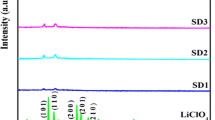
Lithium ion conducting polymer electrolytes based on triblock polymer P(VdCl-co-AN-co-MMA)–LiCl were prepared using a solution casting technique. XRD studies show that the amorphous nature of the polymer electrolyte has been increased due to the addition of LiCl. The maximum amorphous nature has been observed for 40 m% P(VdCl-co-AN-co-MMA)/60 m% LiCl samples. The FTIR study of the lithium ion conducting polymer membrane confirms the complex formation between the polymer P(VdCl-co-AN-co-MMA) and LiCl. The lithium ion conductivity is found to be 1.6 × 10−5 Scm−1 for the 40 m% P(VdCl-co-AN-co-MMA)/60 m% LiCl sample at room temperature. This value is found to be greater than that of pure polymer whose conductivity is found to be 1.5 × 10−8 Scm−1. To improve ionic conductivity, ethylene carbonate has been added as a plasticizer to the 40 m% P(VdCl-co-AN-co-MMA)/60 m% LiCl sample. When we add 0.6 m% of ethylene carbonate, it has been observed that the lithium ion conductivity has increased to 1.3 × 10−3 Scm−1. This value is two orders of magnitude greater than the 40 m% P(VdCl-co-AN-co-MMA)/60 m% LiCl sample. It is also observed from XRD patterns of 40 m% P(VdCl-co-AN-co-MMA)/60 m % LiCl/0.6 m % EC that the amorphous nature has been increased further. A dielectric study has been performed for the above membranes.







Similar content being viewed by others
References
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non-lithium based solid state batteries. J Power Sources 282:299–322
Long L, Wang S, Xiao S, Meng Y (2016) Polymer electrolytes for lithium polymer batteries. J Mater Chem A. doi:10.1039/C6TA02621D
Xue Z, He D, Xie X (2015) Poly(ethylene oxide)-based electrolytes for lithiumion batteries. J Mater Chem A 3:19218
Inbavalli D, Selvasekarapandian S, Sanjeeviraja C, Baskaran R, Nithya S, Kawamura J, Masuda Y (2013) Analysis of lithium ion conducting P(VdCl-co-AN-co-MMA)-LiClO4-DMF triblock copolymer electrolytes. Indian J Appl Res 3(12):498–505
Inbavalli D, Selvasekarapandiyan S, Sanjeeviraja C, Baskaran R, Nithya S, Kawamura J, Masuda Y (2013) Structural, thermal, morphological and electrical analysis of proton conducting tri block copolymer electrolyte P(VdCl-co-AN-co-MMA). International Journal of Electro active materials 1:71–78
Inbavalli D, Selvasekarapandiyan S, Sanjeeviraja C, Baskaran R, Nithya S, Kawamura J, Masuda Y (2015) Analysis of P(VdCl-co-AN-co-MMA)-LiClO4-EC triblock copolymer electrolytes. Bull Mater Sci 38(1):183–190
Meyers RA (ed) (2000) Interpretation of infrared spectra, a practical approach, John Coates in Encyclopedia of analytical chemistry. Wiley, Chichester, pp 10815–10837
Boukamp BA (1986) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20:31–44
Dyre JC (1991) Some remarks on ac conduction in disordered solids. J Non-Cryst Solids 135:219–226
Chen-Yang YW, Chen HC, Lin FJ, Liao CW, Chen TL (2003) Preparation and conductivity of the composite polymer electrolytes based on poly [bis (methoxylethoxyethoxy) phosphazene], LiClO4 and a-Al2O3. Solid State Ionics 156:383–392
Liao CS, Ye WB (2004) Electrochim. Acta 49:4993
Vijaya N, Selvasekarapandian S, Hirankumar G, Karthikeyan S, Nithya H, Ramya CS, Prabu M (2012) Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly(N-vinylpyrrolidone). Ionics 18:91–99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anbazhakan, K., Selvasekarapandiyan, S., Monisha, S. et al. Lithium ion conductivity and dielectric properties of P(VdCl-co-AN-co-MMA)-LiCl-EC triblock co-polymer electrolytes. Ionics 23, 2663–2668 (2017). https://doi.org/10.1007/s11581-016-1957-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1957-x