Abstract
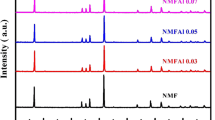
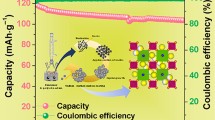
Layered P2-Na0.67Al x Co 1-x O2 (0.0 ≤ × ≤ 0.5) nanopowders were prepared by solution combustion process. These nanopowders were characterized by thermogravimetric analysis (TGA), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, Raman spectroscopy and field emission scanning electron microscopy (FE-SEM) analysis. The electrochemical behaviours of the prepared layered P2-Na0.67Al x Co 1-x O2 nanopowder-based electrodes were investigated by cyclic voltammetry, electrochemical impedances and galvanostatic charge-discharge studies in 1 M Na2SO4 solution. The layered P2-Na0.67Al0.3Co0.7O2 has achieved a high specific capacitance of 260 F g−1 at a constant current density of 1 A g−1. The excellent electrochemical performance of Na0.67Al0.3Co0.7O2 is due to the partial substitution of Al3+ ions for Co3+ ions that leads to decrease in lattice parameter, resulting in the better structural stability during the Na+ ion intercalation/deintercalation reaction process. Moreover, the Na0.67Al0.3Co0.7O2 demonstrates a long cycle life with 80.1 % of its specific capacitance retention even after 5000 continuous charge-discharge process at a constant current density of 1 A g−1.








Similar content being viewed by others
References
Jian Z, Raju V, Li Z, Xing Z, YS H, Xiulei J (2015) A high-power symmetric Na-ion pseudocapacitor. Adv Funct Mater 25:5778–5785
Aravindan V, Ulaganathan M, Madhavi S (2016) Research progress in Na-ion capacitors. J Mater Chem A 4:7538–7548
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22:587–603
Fergus JW (2010) Recent developments in cathode materials for lithium ion batteries. J Power Sources 195:939–954
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5:5884–5901
Kim S-W, Seo D-H, Ma X, Ceder G, Kang K (2012) Electrode materials for rechargeable sodium-ion batteries: potential alternatives to current lithium-ion batteries. Adv Energy Mater 2:710–721
Lee DH, Xu J, Meng YS (2013) An advanced cathode for Na-ion batteries with high rate and excellent structural stability. Phys Chem Chem Phys 15:3304–3312
Chen Z, Augustyn V, Jia X, Xiao Q, Dunn B, Lu Y (2012) High-performance sodium-ion pseudocapacitors based on hierarchically porous nanowire composites. ACS Nano 6:4319–4327
Ding R, Qi L, Wang H (2013) An investigation of spinel NiCo2O4 as anode for Na-ion capacitors. Electrochim Acta 114:726–735
Lu K, Song B, Gao X, Dai H, Zhang J, Ma H (2016) High-energy cobalt hexacyanoferrate and carbon micro-spheres aqueous sodium-ion capacitors. J Power Sources 303:347–353
Lu K, Li D, Gao X, Dai H, Wang N, Ma H (2015) An advanced aqueous sodium-ion supercapacitor with a manganous hexacyanoferrate cathode and a Fe3O4/rGO anode. J Mater Chem A 3:16013–16019
Zhao J, Zhao L, Dimov N, Okada S, Nishida T (2013) Electrochemical and thermal properties of α-NaFeO2 cathode for Na-ion batteries. J Electrochem Soc 160:A3077–A3081
Ding J-J, Zhou Y-N, Sun Q, Z-W F (2012) Cycle performance improvement of NaCrO2 cathode by carbon coating for sodium ion batteries. Electrochem Commun 22:85–88
Berthelot R, Carlier D, Delmas C (2011) Electrochemical investigation of the P2–NaxCoO2 phase diagram. Nat Mater 10:74–80
Zhou X, Guduru RK, Mohanty P (2013) Synthesis and characterization of Na0.44MnO2 from solution precursors. J Mater Chem A 1:2757
Olszewski W, Ávila Pérez M, Marini C, Paris E, Wang X, Iwao T, Okubo M, Yamada A, et al. (2016) Temperature dependent local structure of NaxCoO2 cathode material for rechargeable sodium-ion batteries. J Phys Chem C 120:4227–4232
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-Ion Batteries. Adv Funct Mater 23:947–958
Thorne JS, Dunlap RA, Obrovac MN (2012) Structure and electrochemistry of NaxFexMn1-xO2 (1.0 < =x < =0.5) for Na-ion battery positive electrodes. J Electrochem Soc 160:A361–A367
Delmas C, Fouassier C, Hagenmuller P (1980) Structural classification and properties of the layered oxides. Phys B 99:81–85
Yuan D, He W, Pei F, Wu F, Wu Y, Qian J, Cao Y, Ai X, et al. (2013) Synthesis and electrochemical behaviors of layered Na0.67[Mn0.65Co0.2Ni0.15]O2 microflakes as a stable cathode material for sodium-ion batteries. J Mater Chem A 1:3895
Wang X, Tamaru M, Okubo M, Yamada A (2013) Electrode properties of P2 − Na2/3 MnyCo1−yO2 as cathode materials for sodium-ion batteries. J Phys Chem C 117:15545–15551
Shacklette LW, Jew TR, Townsend L (1985) Rechargeable electrodes from sodium cobalt bronzes. J Electrochem Soc 135:2669–2674
Qu QT, Shi Y, Tian S, Chen YH, Wu YP, Holze R (2009) A new cheap asymmetric aqueous supercapacitor: activated carbon//NaMnO2. J Power Sources 194:1222–1225
Zhang BH, Liu Y, Chang Z, Yang YQ, Wen ZB, Wu YP (2014) Nanowire K0.19MnO2 from hydrothermal method as cathode material for aqueous supercapacitors of high energy density. Electrochim Acta 130:693–698
Zhang BH, Liu Y, Chang Z, Yang YQ, Wen ZB, Wu YP, Holze R (2014) Nanowire Na0.35MnO2 from a hydrothermal method as a cathode material for aqueous asymmetric supercapacitors. J Power Sources 253:98–103
Hou Y, Tang H, Li B, Chang K, Chang Z, Yuan XZ, Wang H (2016) Hexagonal-layered Na0.7MnO2.05 via solvothermal synthesis as an electrode material for aqueous Na-ion supercapacitors. Mater Chem Phys 171:137–144
Saradha T, Subramania A, Balakrishnan K, Muzhumathi S (2015) Microwave-assisted combustion synthesis of nanocrystalline Sm-doped La2Mo2O9 oxide-ion conductors for SOFC application. Mater Res Bull 68:320–325
Kumar M, Subramania A, Balakrishnan K (2014) Preparation of electrospun Co3O4 nanofibers as electrode material for high performance asymmetric supercapacitors. Electrochim Acta 149:152–158
Valefi M, Falamaki C, Ebadzadeh T, Hashjin MS (2007) New insights of the glycine-nitrate process for the synthesis of nano-crystalline 8YSZ. J Am Ceram Soc 90:2008–2014
Liu X, Zhang N, Ni J, Gao L (2013) Improved electrochemical performance of sol–gel method prepared Na4Mn9O18 in aqueous hybrid Na-ion supercapacitor. J Solid State Electrochem 17:1939–1944
Lei Y, Li X, Liu L, Ceder G (2014) Synthesis and stoichiometry of di ff erent layered sodium cobalt oxides. Chem Mater 26:5288–5296
Baster D, Dybko K, Szot M, Świerczek K, Molenda J (2014) Sodium intercalation in NaxCoO2−y—correlation between crystal structure, oxygen nonstoichiometry and electrochemical properties. Solid State Ionics 262:206–210
Elumalai P, Vasan HN, Munichandraiah N (2004) Microwave synthesis and electrochemical properties of LiCo1−xMxO2 (M = Al and Mg) cathodes for Li-ion rechargeable batteries. J Power Sources 125:77–84
Shi Y, Liu Y, Yang H, Nie C, Jin R, Li J (2004) Raman spectroscopy study of NaxCoO2 and superconducting NaxCoO2∙yH2O. Phys Rev B 70:052502
Pétrissans X, Bétard A, Giaume D, Barboux P, Dunn B, Sicard L, Piquemal J-Y (2012) Solution synthesis of nanometric layered cobalt oxides for electrochemical applications. Electrochim Acta 66:306–312
Balakrishnan K, Kumar M, Subramania A (2014) Synthesis of polythiophene and its carbonaceous nanofibers as electrode materials for asymmetric supercapacitors. Adv Mater Res 938:151–157
Khoo E, Wang J, Ma J, Lee PS (2010) Electrochemical energy storage in a β-Na0.33V2O5 nanobelt network and its application for supercapacitors. J Mater Chem 20:8368
Zhang B-H, Yu F, Zhang L, Wang X, Wen Z, Wu Y-P, Holze R (2014) Na0.35MnO2 /CNT nanocomposite from a hydrothermal method as electrode material for aqueous supercapacitors. Zeitschrift für Anorg und Allg Chemie 640:2908–2913
Mai L, Li H, Zhao Y, Xu L, Xu X, Luo Y, Zhang Z, Ke W, et al. (2013) Fast ionic diffusion-enabled Nanoflake electrode by spontaneous electrochemical pre-intercalation for high-performance supercapacitor. Sci Rep 3:1–8
Zhu H, Lee KT, Hitz GT, Han X, Li Y, Wan J, Lacey S, Cresce AVW, et al. (2014) Free-standing Na2/3Fe1/2Mn1/2O2@Graphene film for a sodium-ion battery cathode. ACS Appl Mater Interfaces 6:4242–4247
Acknowledgments
The authors gratefully acknowledge the DST-Nano Mission, New Delhi (SR/NM/NS-25/2010) and UGC, New Delhi (41-1002/ 2012 SR) for the financial supports. A part of this research work has been presented in the 4th International Conferences on Advances in Energy Research (ICAER-2013) held at IIT-B, Mumbai, India, on Dec. 10-12, 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, K., Kirubasankar, B. & Angaiah, S. Synthesis and electrochemical performance of P2-Na0.67AlxCo1-xO2 (0.0 ≤ × ≤ 0.5) nanopowders for sodium-ion capacitors. Ionics 23, 731–739 (2017). https://doi.org/10.1007/s11581-016-1821-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-016-1821-z