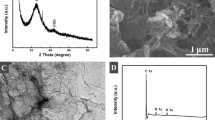
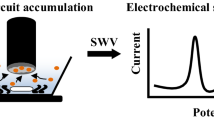
Abstract
A simple, sensitive, and reliable carboxylic group functionalized single-walled carbon nanotubes (f-SWCNTs)/poly(hydroxymethylated-3,4-ethylenedioxythiophene) (PEDOTM) modified glassy carbon electrode (GCE) was successfully developed for the electrochemical determination of catechin (CAT). In view of the merits of extraordinary conductivity of PEDOTM and excellent electrocatalytic property of f-SWCNTs, the f-SWCNTs/PEDOTM/GCE modified electrode exhibited a strong electrocatalytic activity for the oxidation of CAT. Under optimized conditions, the proposed modified electrode showed a wide linear response for CAT in the concentration range between 0.039 and 40.84 μM, with a low detection limit of 0.013 μM. Furthermore, the modified electrode also exhibited a good reproducibility and long-term stability, as well as high selectivity, which also was a good candidate for the electrochemical detection and analysis of CAT in commercial green tea.









Similar content being viewed by others
References
Janeiro P, Bret AMO (2004) Catechin electrochemical oxidation mechanisms. Anal Chim Acta 518:109–115
Arts ICW, Hollman PCH (1998) Optimization of a quantitative method for the determination of catechins in fruits and legumes. J Agric Food Chem 46:5156–5162
Yang L-J, Cheng T, Xiong H-Y, Zhang X-H, Wan S-F (2009) Electrochemical properties of catechin at a single-walled carbon nanotubes–cetylramethylammonium bromide modified electrode. Bioelectrochemistry 75:158–162
Cook NC, Samman S (1996) Flavonoids: chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76
El-Hady D, El-Maali N (2008) Selective square wave voltammetric determination of (1)-catechin in commercial tea samples using beta-cyclodextrin modified carbon paste electrode. Microchim Acta 161:225–231
Higdona JV, Frei B (2003) Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43(1):89–143
Fukumoto LR, Mazza G (2000) Assessing antioxidant and prooxidant activities of phenolic compounds. J Agric Food Chem 48(8):3597–3604
Wang R, Zhou W, Jiang X (2008) Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J Food Eng 87:505–513
Moccelini SK, Fernandes SC, de Camargo TP, Ademir N, Iolanda Cruz V (2009) Self-assembled monolayer of nickel (II) complex and thiol on gold electrode for the determination of catechin. Talanta 78:1063–1068
Ozyurt D, Demirata B, Apak R (2007) Determination of total antioxidant capacity by a new spectrophotometric method based on Ce(IV) reducing capacity measurement. Talanta 71:1155–1165
Manabu M, Shin-ichi Y, Kenji K, Tokuji I (2002) Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim Biophys Acta Gen Subj 1569:35–44
Chen Z, Zhang L, Chen G (2008) Microwave-assisted extraction followed by capillary electrophoresis-amperometric detection for the determination of antioxidant constituents in Folium Eriobotryae. J Chromatogr A 1193:178–181
El-Hady DA, El-Maali NA (2008) Determination of catechin isomers in human plasma subsequent to green tea ingestion using chiral capillary electrophoresis with a high-sensitivity cell. Talanta 76:138–145
Tsukagoshi K, Taniguchi T, Nakajima R (2007) Analysis of antioxidants using a capillary electrophoresis with chemiluminescence detection system. Anal Chim Acta 589:66–70
Dhalwal K, Shinde VM, Biradar YS, Mahadik KR (2008) Simultaneous quantification of bergenin, catechin, and gallic acid from Bergenia ciliata and Bergenia ligulata by using thin-layer chromatography. J Food Compos Anal 21:496–500
Khokhar S, Venema D, Hollman PCH, Dekker M, Jongen W (1997) A RP-HPLC method for the determination of tea catechins. Cancer Lett 114:171–172
Iacopini P, Baldi M, Storchi P, Sebastiani L (2008) Catechin, epicatechin, quercetin, rutin and resveratrol in red grape: content, in vitro antioxidant activity and interactions. J Food Compos Anal 21:589–598
Wang H, Provan GJ, Helliwell K (2003) Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem 87:307–311
Wu J, Wang H, Liu F, Chen Z, Jiang JH, Shen G, Yu R (2005) Detection of catechin based on its electrochemical autoxidation. Talanta 65:511–517
El-Hady DA (2007) Selective and sensitive hydroxypropyl-beta-cyclodextrin based sensor for simple monitoring of (+)-catechin in some commercial drinks and biological fluids. Anal Chim Acta 593:178–187
Jarosz-Wilkołazka A, Ruzgas T, Gorton L (2004) Use of laccase-modified electrode for amperometric detection of plant flavonoids. Enzym Microb Technol 35:238–241
Nie G, Li C, Lin Z, Wang L (2014) Fabrication of a simple and sensitive QDs-based electrochemiluminescence immunosensor using a nanostructured composite material for the detection of tumor markers alpha-fetoprotein. J Mater Chem B 2:8321–8328
Nie G, Bai Z, Chen J, Yu W (2012) Simple label-free femtomolar DNA detection based on a nanostructure composite material: MWNT-doped poly(indole-6-carboxylic acid). ACS Macro Lett 1:1304–1307
Lin Z, Li C, Zhao D, Wu T, Nie G (2014) An electrochemical immunosensor for the tumor marker α-fetoprotein using a glassy carbon electrode modified with a poly(5-formylindole), single-wall carbon nanotubes, and coated with gold nanoparticles and antibody. Microchim Acta 181:1601–1608
Groenendaal LB, Jonas F, Freitag D, Pielartzik H, Reynolds JR (2000) Poly(3,4-ethylenedioxythiophene) and its derivatives: past, present, and future. Adv Mater 12:481–494
Elschner A, Kirchmeyer S, Lovenich W, Merker U, Reuter K (2011) PEDOT: principles and applications of an intrinsically conductive polymer. CRC Press, London
Jang J, Chang M, Yoon H (2005) Chemical sensors based on highly conductive poly(3,4-ethylenedioxythiophene) nanorods. Adv Mater 17:1616–1620
Wu L, Lu L, Zhang L, Xu J, Zhang K, Wen Y, Duan X, Yang F (2013) Electrochemical determination of the anticancer herbal drug shikonin at a nanostructured poly(hydroxymethylated-3,4-ethylenedioxythiophene) modified electrode. Electroanalysis 25:2244–2250
Stéphan O, Schottland P, Le Gall P-Y, Chevrot C, Mariet C, Carrier M (1998) Flectrochemical behaviour of 3,4-ethylenedioxythiophene functionalized by a sulphonate group. Application to the preparation of poly(3,4-ethylenedioxythiophene) having permanent cation-exchange properties. J Electroanal Chem 443:217–226
Lima A, Schottland P, Sadki S, Chevrot C (1998) Electropolymerization of 3,4-ethylenedioxythiophene and 3,4-ethylenedioxythiophene methanol in the presence of dodecylbenzenesulfonate. Synth Met 93:33–41
Xiao Y, Cui X, Hancock JM, Bouguettaya M, Reynolds JR, Martin DC (2004) Electrochemical polymerization of poly(hydroxymethylated-3,4-ethylenedioxythiophene) (PEDOT-MeOH) on multichannel neural probes. Sensors Actuators B 99:437–443
Wen Y, Dong L, Lu Y, He H, Xu J, Duan X, Liu M (2012) Poly(3,4-ethylenedioxythiophene methanol)/ascorbate oxidase/nafion-single-walled carbon nanotubes biosensor for voltammetric detection of vitamin C. Chin J Polym Sci 30:824–836
Feng Z, Yao Y, Xu J, Zhang L, Wang Z, Wen Y (2014) One-step co-electrodeposition of graphene oxide doped poly(hydroxymethylated-3,4-ethylenedioxythiophene) film and its electrochemical studies of indole-3-acetic acid. Chin Chem Lett 25:511–516
Yao Y, Zhang L, Wang Z, Xu J, Wen Y (2014) Electrochemical determination of quercetin by self-assembled platinum nanoparticles/poly(hydroxymethylated-3,4-ethylenedioxylthiophene) nanocomposite modified glassy carbon electrode. Chin Chem Lett 25:505–510
Zhang L, Wen Y, Yao Y, Xu J, Duan X, Zhang G (2014) Synthesis and characterization of PEDOT derivative with carboxyl group and its chemo/bio sensing application as nanocomposite, immobilized biological and enhanced optical materials. Electrochim Acta 116:343–354
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications, 2nd edn. Wiley, New York
Yu YG, Cao DY, Luo X, Tang Y, Li H (2012) Sensitivity and selectivity determination of bisphenol A using SWCNT–CD conjugate modified glassy carbon electrode. J Hazard Mater 199–200:111–118
Zhou C, Liu Z, Dong Y, Li D (2009) Electrochemical behavior of o-nitrophenol at hexagonal mesoporous silica modified carbon paste electrodes. Electroanalysis 21:853–858
Yao Y, Wen Y, Zhang L, Wang Z, Zhang H, Xu J (2014) Electrochemical recognition and trace-level detection of bactericide carbendazim using carboxylic group functionalized poly(3,4-ethylenedioxythiophene) mimic electrode. Anal Chim Acta 831:38–49
Laviron E (1974) Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. J Electroanal Chem Interfacial Electrochem 52:355–393
Anson FC (1964) Application of potentiostatic current integration to the study of the adsorption of cobalt (III)-(ethylenedinitrilo (tetraacetate) on mercury electrodes, Anal Chem 36(4):932–934
Velasco JG (1997) Determination of standard rate constants for electrochemical irreversible processes from linear sweep voltammograms. Electroanalysis 9:880–882
Acknowledgments
The authors would like to acknowledge the financial support of this work by the National Natural Science Foundation of China (No. 51463008, 51272096, and 51263010), GanPo Outstanding Talents 555 Projects, Jiangxi Provincial Department of Education (No. GJJ12595, GJJ13565), Youth Science and Technology Talent Training Plan of Chongqing Science and Technology Commission (cstc2014kjrc-qnrc10006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yuanyuan Yao and Long Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yao, Y., Zhang, L., Wen, Y. et al. Voltammetric determination of catechin using single-walled carbon nanotubes/poly(hydroxymethylated-3,4-ethylenedioxythiophene) composite modified electrode. Ionics 21, 2927–2936 (2015). https://doi.org/10.1007/s11581-015-1494-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1494-z