Abstract
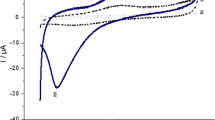
Poly(3,4-ethylenedioxythiophene) (PEDOT) modified with ferrocene carboxylic acid (FC1), ferrocene (FC2), and cobaltocene (CC) is introduced as a sensor electrode. FC or CC was “sandwiched” between two layers of PEDOT in the presence of sodium dodecyl sulfate (PEDOT/mediator/PEDOT…SDS). The composite electrodes were evaluated for the electrocatalytic oxidation of dopamine (DA). The FC1 mediator showed the highest rate for electron transfer and enhanced electrocatalytic activity. This is due to the inclusion of the ferrocenium ion and the polar substituted –COOH group in the matrix which increases the electronic conduction of the film. SDS enhanced the preconcentration/accumulation of DA ions at the surface that resulted in enhanced detection. Detection limit in human urine was 0.069 μmol L−1 in the linear dynamic range of 6–300 μmol L−1, with satisfying recovery results. The PEDOT/FC1/PEDOT…SDS composite was used for simultaneous determination of dopamine in the presence of ascorbic acid (AA) and uric acid (UA).







Similar content being viewed by others
References
Atta NF, Galal A, Ahmed RA (2011) Bioelectrochemistry 80:132–141
Atta NF, Galal A, El-Ads EH (2012) Electrochim Acta 69:102–111
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007) Talanta 72:976–983
Hu G, Zhang D, Wu W, Yang Z (2008) Colloids Surf B 62:199–205
Goyal RN, Aliumar A, Oyama M (2009) J Electroanal Chem 631:58–61
Li J, Lin X (2007) Sens Actuators B 124:486–493
Kros A, Sommerdijk NAJM, Nolte RJM (2005) Sens Actuators B 106:289–295
Atta NF, El-Kady MF (2010) Sens Actuators B 145:299–310
Mathiyarasu J, Senthilkumar S, Phani KLN, Yegnaraman V (2008) Mater Lett 62:571–573
Selvaganesh SV, Mathiyarasu J, Phani KLN, Yegnaraman V (2007) Nanoscale Res Lett 2:546–249
Namboothiry MAG, Zimmerman T, Coldren FM, Liu J, Kim K, Carroll DL (2007) Synth Met 157:580–584
Kim BY, Cho MS, Kim YS, Son Y, Lee Y (2005) Synth Met 153:149–152
Harish S, Mathiyarasu J, Phani KLN (2009) Mater Res Bull 44:1828–1833
Zanardi C, Terzi F, Seeber R (2010) Sens Actuators B 148:277–282
Galal A (1998) J Solid State Electrochem 2:7–15
Yaghoubian H, Karimi-Maleh H, Khailzadeh MA, Karimi F (2009) J Serb Chem Soc 74(12):1443–1453
Yaghoubian H, Karimi-Maleh H, Khalilzadeh MA, Karimi F (2009) Int J Electrochem Sci 4:993–1003
Skeika T, Zuconelli CR, Fujiwara ST, Pessoa CA (2011) Sensors 11(2):1361–1374
Radhakrishnan S, Paul S (2007) Sens Actuators B 125:60–65
Şenel M (2011) Synth Met 161(17–18):1861–1868
Atta NF, Galal A, Wassel AA, Ibrahim AH (2012) Int J Electrochem Sci 7:10501–10518
Galal A, Atta NF, Darwish SA, Abdallah AM (1997) Bull Chem Soc Jpn 70:1769–1776
Connelly NG, Geiger WE (1996) Chem Rev 96:877–910
Atta NF, Darwish SA, Khalil SE, Galal A (2007) Talanta 72(4):1438–1445
Atta NF, Galal A, Abu-Attia FM, Azab SM (2011) Electrochim Acta 56:2510–2517
Atta NF, Galal A, El-Ads EH (2012) Analyst 137:2658–2668
Galal A, Atta NF, El-Ads EH (2012) Talanta 93:264–273
Fernandez L, Carrero H (2005) Electrochim Acta 50(5):1233–1240
Greenwood NN, Earnshaw A (1984) P. Press, Oxford 19:1261-1316
Wen XL, Hua YH, Liu AL (1999) Talanta 50:1027–1033
Zhang F, Dryhurst G (1993) Bioorg Chem 21:392–410
Chen Y, Guo L-R, Chen W, Yang X-J, Jin B, Zheng L-M, Xia X-H (2009) Bioelectrochemistry 75:26–31
Acknowledgments
The authors would like to acknowledge the financial support from Cairo University through the Office of the President for Research Funds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Atta, N.F., Galal, A., Ali, S.M. et al. Electrochemistry and detection of dopamine at a poly(3,4-ethylenedioxythiophene) electrode modified with ferrocene and cobaltocene. Ionics 21, 2371–2382 (2015). https://doi.org/10.1007/s11581-015-1417-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-015-1417-z