Abstract
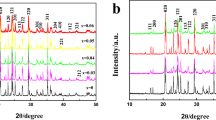
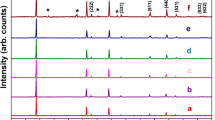
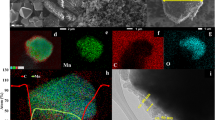
LiCr x Mn2−x O4 (x = 0, 0.02, 0.05, 0.12) materials were effectively synthesized by imidazolium-based ionic liquid as reaction medium at ambient pressure. The morphologies of Cr-doped LiMn2O4 via calcination were characterized by scanning electron microscopy (SEM). SEM reveals that the LiCr0.12Mn1.88O4 sample has regular nanostructure and a uniform particle size of 50–100 nm. Among the four samples prepared in ionic liquid, the charge/discharge tests indicate that LiCr0.12Mn1.88O4 presents the best performance of rate capacity and cycle stability. A typical LiCr0.12Mn1.88O4 delivers the initial discharge capacity of 129.6 mAh g−1 and behaves a quite slow capacity fading with 96.8 % of initial capacity remained after 200 cycles at 0.5 C in the voltage range of 3.4–4.3 V. The improved electrochemical performance can be attributed to Cr doping and recyclable ionothermal method. Furthermore, this ionothermal synthesis is believed to provide a new reaction route for lithium-ion battery materials with mild reaction conditions.







Similar content being viewed by others
References
He L, Zhang S, Wei X, Du Z, Liu G, Xing Y (2012) Synthesis and electrochemical performance of spinel-type LiMn2O4 using γ-MnOOH rods as self-template for lithium ion battery. J Power Sources 220:228–235
Aricò AS, Bruce P, Scrosati B, Tarascon JM, Van Schalkwijk W (2005) Nanostructured materials for advanced energy conversion and storage devices. Nat Mater 4:366–377
Meng H, Huang B, Yin J, Yao X, Xu X (2014) Synthesis and electrochemical properties of LiNi1/3Co1/3Mn1/3O2 cathodes in lithium-ion and all-solid-state lithium batteries. Ionics. doi:10.1007/s11581-014-1152-x
Fey GTK, Lu CZ, Prem Kumar T (2003) Solid-state synthesis and electrochemical characterization of LiMyCr0.5-yMn1.5O4(M = Fe or Al; 0.0 < y < 0.4) spinels. Mater Chem Phys 80:309–318
Wang J, Zhang Q, Li X, Wang Z, Zhang K, Guo H, Yan G, Huang B, He Z (2013) A graphite functional layer covering the surface of LiMn2O4 electrode to improve its electrochemical performance. Electrochem Commun 36:6–9
Wang J, Zhang Q, Li X, Wang Z, Guo H, Xu D, Zhang K (2014) Sputtering graphite coating to improve the elevated-temperature cycling ability of the LiMn2O4 electrode. Phys Chem Chem Phys 16:16021–16029
Zhou WJ, Bao SJ, He BL, Liang YY, Li HL (2006) Synthesis and electrochemical properties of LiAl0.05Mn1.95O4 by the ultrasonic assisted rheological phase method. Electrochim Acta 51:4701–4708
Wang J, Li Z, Yang J, Tang J, Yu J, Nie W, Lei G, Xiao Q (2012) Effect of Al-doping on the electrochemical properties of a three-dimensionally porous lithium manganese oxide for lithium-ion batteries. Electrochim Acta 75:115–122
Deng B, Nakamura H, Yoshio M (2008) Capacity fading with oxygen loss for manganese spinels upon cycling at elevated temperatures. J Power Sources 180:864–868
Manthiram A, Murugan AV, Sarkar A, Muraliganth T (2008) Nanostructured electrode materials for electrochemical energy storage and conversion. Energ Environ Sci 1:621–638
Matsuda K, Taniguchi I (2004) Relationship between the electrochemical and particle properties of LiMn2 O4 prepared by ultrasonic spray pyrolysis. J Power Sources 132:156–160
Santhanam R, Rambabu B (2010) Research progress in high voltage spinel LiNi0.5Mn1.5O4 material. J Power Sources 195:5442–5451
Kakuda T, Uematsu K, Toda K, Sato M (2007) Electrochemical performance of Al-doped LiMn2O4 prepared by different methods in solid-state reaction. J Power Sources 167:499–503
Choi HJ, Lee KM, Kim GH, Lee JG (2001) Mechanochemical synthesis and electrochemical properties of LiMn2O4. J Am Ceram Soc 84:242–244
Hwang B, Santhanam R, Liu D (2001) Characterization of nanoparticles of LiMn2O4 synthesized by citric acid sol-gel method. J Power Sources 97:443–446
Mao F, Wu D, Zhou Z, Wang S (2014) Structural and electrochemical properties of LiFe1- 3x/2Bi x PO4/C synthesized by sol-gel. Ionics 20:1665–1669
Song MY, Kwon IH, Park HR, Mumm DR (2011) Electrochemical properties of LiCoyMn2- yO4 synthesized using a combustion method in a voltage range of 3.5-5.0 V. Ceram Int 37:2215–2220
Jiang C, Dou S, Liu H, Ichihara M, Zhou H (2007) Synthesis of spinel LiMn2O4 nanoparticles through one-step hydrothermal reaction. J Power Sources 172:410–415
Recham N, Dupont L, Courty M, Djellab K, Larcher D, Armand M, Tarascon JM (2009) Ionothermal synthesis of tailor-made LiFePO4 powders for Li-ion battery applications. Chem Mater 21:1096–1107
Liu J, Liu X, Kong X, Zhang H (2012) Controlled synthesis, formation mechanism and upconversion luminescence of NaYF4:Yb, Er nano-/submicrocrystals via ionothermal approach. J Solid State Chem 190:98–103
Li XL, Chen JJ, Luo M, Chen XY, Li PP (2010) Quantum chemical calculation of hydroxyalkyl ammonium functionalized ionic liquids for absorbing SO2. Acta Phys -Chim Sin 26:1364–1372
Li XL, He WX, Xiao ZH, Peng FF, Chen JJ (2013) Ionothermal synthesis and rate performance studies of nanostructured Li3V2(PO4)3/C composites as cathode materials for lithium-ion batteries. J Solid State Electrochem 17:1991–2000
Li XL, Guo W, Liu YF, He WX, Xiao ZH (2014) Spinel LiNi0.5Mn1.5O4 as superior electrode materials for lithium-ion batteries: ionic liquid assisted synthesis and the effect of CuO coating. Electrochim Acta 116:278–283
Lee HW, Muralidharan P, Ruffo R, Mari CM, Cui Y, Kim DK (2010) Ultrathin spinel LiMn2O4 nanowires as high power cathode materials for Li-ion batteries. Nano Lett 10:3852–3856
Kim DK, Muralidharan P, Lee HW, Ruffo R, Yang Y, Chan CK, Peng H, Huggins RA, Cui Y (2008) Spinel LiMn2O4 nanorods as lithium ion battery cathodes. Nano Lett 8:3948–3952
Xiao L, Zhao Y, Yang Y, Cao Y, Ai X, Yang H (2008) Enhanced electrochemical stability of Al-doped LiMn2O4 synthesized by a polymer-pyrolysis method. Electrochim Acta 54:545–550
Yang Y, Xie C, Ruffo R, Peng H, Kim DK, Cui Y (2009) Single nanorod devices for battery diagnostics: a case study on LiMn2O4. Nano Lett 9:4109–4114
Yao J, Lv L, Shen C, Zhang P, Aguey-Zinsou KF, Wang L (2013) Nano-sized spinel LiMn2O4 powder fabricated via modified dynamic hydrothermal synthesis. Ceram Int 39:3359–3364
Xia Y, Zhang W, Huang H, Gan Y, Li C, Tao X (2011) Synthesis and electrochemical properties of Nb-doped Li3V2(PO4)3/C cathode materials for lithium-ion batteries. Mater Sci Eng B 176:633–639
Qiao Y, Tu J, Wang X, Zhang J, Yu Y, Gu C (2011) Self-assembled synthesis of hierarchical waferlike porous Li-V-O composites as cathode materials for lithium ion batteries. J Phys Chem C 115:25508–25518
Xiang J, Tu J, Zhang L, Wang X, Zhou Y, Qiao Y, Lu Y (2010) Improved electrochemical performances of 9LiFePO(4) center dot Li3V2(PO4)/C composite prepared by a simple solid-state method. J Power Sources 195:8331–8335
Huang J, Yang L, Liu K, Tang Y (2010) Synthesis and characterization of Li3V(2- 2x/3)Mgx(PO4)3/C cathode material for lithium-ion batteries. J Power Sources 195:5013–5018
Sehrawat R, Sil A (2014) Polymer gel combustion synthesis of LiFePO4/C composite as cathode material for Li-ion battery. Ionics. doi:10.1007/s11581-014-1229-6
Yao J, Shen C, Zhang P, Ma CA, Gregory DH, Wang L (2013) Spinel-Li3.5+xTi5O12 coated LiMn2O4 with high surface Mn valence for an enhanced cycling performance at high temperature. Electrochem Commun 31:92–95
Jiang RY, Cui CY, Ma HY (2013) Poly(vinyl pyrrolidone)-assisted hydrothermal synthesis of LiMn2O4 nanoparticles with excellent rate performance. Mater Lett 91:12–15
Acknowledgments
This work was supported by the Science and Technology Project of Anhui Province (1301022077) and the Science and Technology Project of Land and Resources of Anhui Province (2012-k-18).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Zhou, Q., Wang, H. et al. Ionothermal synthesis and enhanced electrochemical performance of nanostructure Cr-doped LiMn2O4 for lithium-ion batteries. Ionics 21, 1517–1523 (2015). https://doi.org/10.1007/s11581-014-1352-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1352-4