Abstract
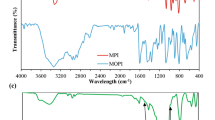
To improve the copper corrosion resistance of epoxy-functionalized hybrid sol–gel monolayers (Hy) consisting of 3-glycidoxypropyltrimethoxysilane (GPTMS-functional monomer) and tetraethoxysilane (TEOS-reticulating agent), the copper surface was modified by a well-defined inhibitor viz., thiosemicarbazide (TSC). At first, the copper surface was activated by TSC monolayers through Cu–S bonds which end up with free tail amino groups. Secondly, Hy monolayers were immobilized on the TSC-modified copper surface via epoxy-amine reaction. The interaction of TSC with copper and Hy was investigated by Fourier transform infrared spectroscopy, which revealed the cleavage of epoxy ring due to the cross-linking reaction with free amino groups of TSC monolayers. The surface morphology of these monolayers was investigated by scanning electron microscopy and atomic force microscopy. The effectiveness of TSC and Hy monolayers on corrosion protection of copper was scrutinized by electrochemical methods viz., potentiodynamic polarization studies and electrochemical impedance spectroscopy techniques. These studies revealed the enhancement of copper corrosion resistance of epoxy-functionalized Hy monolayers by the TSC activation.









Similar content being viewed by others
References
Snihirova D, Lamaka SV, Taryba M, Salak AN, Kallip S, Zheludkevich ML, Ferreira MGS, Montemor MF (2010) Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Appl Mater Interf 2:3011–3022
Biswas BN, Mollah MYA, Susan MABH (2012) Potentiodynamic studies on corrosion of copper by chloride ions and its inhibition by inorganic and organic ions in aqueous buffer solution. Ionics 18:189–195
Liang C, Wang P, Wu B, Huang N (2010) Inhibition of copper corrosion by self-assembled monolayers of triazole derivative in chloride-containing solution. J Solid State Electrochem 14:1391–1399
Younis AA, El-Sabbah MMB, Holze R (2012) The effect of chloride concentration and pH on pitting corrosion of AA7075 aluminum alloy coated with phenyltrimethoxysilane. J Solid State Electrochem 16:1033–1040
Ma HY, Yang C, Chen SH, Jiao YL, Huang SX, Li DG, Luo JL (2003) Electrochemical investigation of dynamic interfacial processes at 1-octadecanethiol-modified copper electrodes in halide-containing solutions. Electrochim Acta 48:4277–4289
Ulman A (1996) Formation and structure of self-assembled monolayers. Chem Rev 96:1533–1554
Schreiber F (2000) Structure and growth of self-assembling monolayers. Prog Surf Sci 65:151–257
Jennings GK, Munro JC, Yong TH, Laibinis PE (1998) Effect of chain length on the protection of copper by n-alkanethiols. Langmuir 14:6130–6139
Laibinis PE, Whitesides GM (1992) Self-assembled monolayers of n-alkanethiolates on copper are barrier films that protect the metal against oxidation by air. J Am Chem Soc 114:9022–9028
Silva DPB, Neves RS, Motheo AJ (2010) Electrochemical behaviour of the AA2024 aluminium alloy modified with self-assembled monolayers/polyaniline double films. Mol Cryst Liq Cryst 521:179–186
Maege I, Jaehne E, Henke A, Adler HJP, Bram C, Jung C, Stratmann M (1997) Self-assembling adhesion promoters for corrosion resistant metal polymer interfaces. Prog Org Coat 34:1–12
Cecchetto L, Denoyelle A, Delabouglise D, Petit JP (2008) A silane pre-treatment for improving corrosion resistance performances of emeraldine base-coated aluminium samples in neutral environment. Appl Surf Sci 254:1736–1743
Li ZF, Ruckenstein E (2002) Strong adhesion and smooth conductive surface via graft polymerization of aniline on a modified glass fiber surface. J Colloid Interf Sci 251:343–349
Jennings GK, Laibinis PE (1996) Self-assembled monolayers of alkanethiols on copper provide corrosion resistance in aqueous environments. Colloids Surf A 116:105–114
Feng YQ, Teo WK, Siow KS, Gao ZQ, Tan KL, Hsieh AK (1997) Corrosion protection of copper by a self‐assembled monolayer of alkanethiol. J Electrochem Soc 144:55–64
Taneichi D, Haneda R, Aramaki K (2001) A novel modification of an alkanethiol self-assembled monolayer with alkylisocyanates to prepare protective films against copper corrosion. Corros Sci 43:1589–1600
Hukovic MM, Babic R, Petrovic Z, Posavecm D (2007) Copper protection by a self-assembled monolayer of alkanethiol: Comparison with benzotriazole. J Electrochem Soc 154:C138–C143
Caprioli F, Beccari M, Martinelli A, Castro VD, Decker F (2010) Copper protection by self-assembled monolayers of aromatic thiols in alkaline solutions. Phys Chem Chem Phys 11:9230–9238
Quan ZL, Chen SH, Li SL (2001) Protection of copper corrosion by modification of self-assembled films of Schiff bases with alkanethiol. Corros Sci 43:1071–1080
Zhang DQ, He XM, Cai QR, Gao LX, Kim GS (2009) Arginine self-assembled monolayers against copper corrosion and synergistic effect of iodide ion. J Appl Electrochem 39:1193–1198
Wang HM, Akid R, Gobara M (2010) Scratch-resistant anticorrosion sol-gel coating for protection of AZ31 magnesium alloy via a low temperature sol-gel route. Corros Sci 52:2565–2570
Mrad M, Montemor MF, Dhouibi L, Triki E (2012) Deposition of hybrid 3-GPTMS’s film on AA2024-T3: dependence of film morphology and protectiveness performance on coating conditions. Prog Org Coat 73:264–271
Metroke TL, Kachurina O, Knobbe ET (2002) Spectroscopic and corrosion resistance characterization of GLYMO–TEOS Ormosil coatings for aluminum alloy corrosion inhibition. Prog Org Coat 44:295–305
Li GL, Wang XM, Li AJ, Wang WQ, Zheng L (2007) Fabrication and adhesive properties of thin organosilane films coated on low carbon steel substrates. Surf Coat Technol 201:9571–9578
Parkhill RL, Knobbe ET, Donley MS (2001) Application and evaluation of environmentally compliant spray-coated ormosil films as corrosion resistant treatments for aluminum 2024-T3. Prog Org Coat 41:261–265
Zucchi F, Grassi V, Frignani A, Trabanelli G (2004) Inhibition of copper corrosion by silane coatings. Corros Sci 46:2853–2865
Singh MM, Rastogi RB, Upadhyay BN, Yadav M (2003) Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions. Mater Chem Phys 80:283–293
Abdel-wahaab SM, Gomma GK (1986) Adsorption of thiosemicarbazide on copper cathodes. J Chem Technol Biotechnol 36:185–190
Peng S, Zhao W, Li H, Zeng Z, Xue Q, Wu X (2012) Synergistic effect of thiourea in epoxy functionalized silica sol–gel coating for copper protection. Surf Coat Technol 213:175–182
Innochenzi P, Kidchob T (2005) Hybrid organic-inorganic sol-gel materials based on epoxy-amine systems. J Sol-Gel Sci Technol 35:225–235
Clayden J, Greeves N, Warren S, Wothers P (2001) Organic chemistry. Oxford University Press, New York
Silverstein RM, Webster FX (1998) Spectrometric identification of organic compounds. Wiley, Canada
Santhakumari R, Ramamurthi K, Ramesh Babu R, Evans HS, Bhagavannarayana G, Hema R (2011) Growth and characterization of thiosemicarbazide hydrochloride: a semiorganic NLO material. Spectrochim Acta 82:102–107
Lee YM, Chien YHC, Leung MK, Hu CC, Wan CC (2013) Surface protection of copper by self-assembly of novel poly (5-methylenebenzotriazol-N-yl). J Mater Chem A 1:3629–3638
Chen W, Hong S, Li HB, Luo HQ, Li M, Li NB (2012) Protection of copper corrosion in 0.5 M NaCl solution by modification of 5-mercapto-3-phenyl-1,3,4-thiadiazole-2-thione potassium self-assembled monolayers. Corros Sci 61:53–62
Elia A, Wael KD, Dowsett M, Adriaens A (2012) Electrochemical deposition of a copper carboxylate layer on copper as potential corrosion inhibitor. J Solid State Electrochem 16:143–148
Deslouis C, Tribollet B, Mengoli G, Musiani MM (1988) Electrochemical behaviour of copper in neutral aerated chloride solution—I. Steady-state investigation. J Appl Electrochem 18:374–383
Yan CW, Lin HC, Cao CN (2000) Investigation of inhibition of 2-mercaptobenzoxazole for copper corrosion. Electrochim Acta 45:2815–2821
Kear G, Barker BD, Walsh FC (2004) Electrochemical corrosion of unalloyed copper in chloride media—a critical review. Corros Sci 46:109–135
Kılınççeker G, Çelik S (2013) Electrochemical adsorption properties and inhibition of copper corrosion in chloride solutions by ascorbic acid: experimental and theoretical investigation. Ionics 19:1655–1662
Karthik N, Sethuraman MG (2013) A robust method of enhancement of protection ability of electrodeposited silane film over copper surface using H2O2. Mater Corros. doi:10.1002/maco.201307396
Raistrick ID, Franceschetti DR, Macdonald JR (2005) Impedance spectroscopy theory, experiment, and application. Wiley, New York
Amin MA, Khaled KF, Mohsen Q, Arida HA (2010) A study of the inhibition of iron corrosion in HCl solutions by some amino acids. Corros Sci 52:1684–1695
Li X, Deng S, Fu H, Mu G (2010) Synergistic inhibition effect of rare earth cerium(IV) ion and sodium oleate on the corrosion of cold rolled steel in phosphoric acid solution. Corros Sci 52:1167–1178
Trachli B, Keddam M, Takenouti H, Srhiri A (2002) Protective effect of electropolymerized 3-amino 1,2,4-triazole towards corrosion of copper in 0.5 M NaCl. Corros Sci 44:997–1008
Li Y, Fedkiw PS (2007) Effect of gel electrolytes containing silica nanoparticles on aluminum corrosion. Electrochim Acta 52:2471–2477
Macdonald DD (1990) Review of mechanistic analysis by electrochemical impedance spectroscopy. Electrochim Acta 35:1509–1525
Quan XL, Chen SH, Li L, Cui XG (2002) Adsorption behavior of Schiff base and corrosion protection of resulting films to copper substrate. Corros Sci 44:703–715
Sakai RT, da Cruz FMDiL, de Melo HG, Benedetti AV, Santilli CV, Suegama PH (2012) Electrochemical study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA films on tin coated steel in 3.5 % NaCl solution. Prog Org Coat 74:288–301
Fan H, Li S, Zhao Z, Wang H, Shi Z, Zhang L (2011) Inhibition of brass corrosion in sodium chloride solutions by self-assembled silane films. Corros Sci 53:4273–4281
Acknowledgments
The authors are grateful to the Board of Research in Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), Mumbai, Government of India, for the financial support for this work through a major research project on “Sol–Gel” (No. 2011/37C/55/BRNS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karthik, N., Sethuraman, M.G. Improved copper corrosion resistance of epoxy-functionalized hybrid sol–gel monolayers by thiosemicarbazide. Ionics 21, 1477–1488 (2015). https://doi.org/10.1007/s11581-014-1274-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1274-1