Abstract
Phytophthora species are a cause for concern due to their invasive potential and the damage they can cause in agriculture, forestry, and natural ecosystems worldwide. Since water plays a crucial role in their dispersal, stream and river baiting is commonly used to survey risk areas for the presence of quarantine Phytophthora species. However, our understanding of the distribution and diversity of Phytophthora species in European watercourses remains incomplete. This study investigated the presence and diversity of Phytophthora species in Swiss watercourses, with a focus on the highly urbanized Swiss Plateau. Over the period 2012–2016, we sampled 32 watercourses, including major rivers and smaller streams. We isolated Phytophthora on selective media and sequenced the internal transcribed spacer region to identify the species. We recovered 241 Phytophthora isolates, representing 11 species from five major clades. Phytophthora clade 6 prevailed, with P. lacustris being the most common, found in 94.7% of the watercourses. The number of Phytophthora species per watercourse ranged from one to five, with no correlation to watercourse complexity. Our study reveals the presence of six previously unreported species in Switzerland, while known invasive species were not found. Watercourses appear less suited to detect invasive pathogenic Phytophthora species with a still limited distribution in the environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytophthora is a genus of fungal-like oomycetes whose number of species has nearly doubled in the last decade (Brasier et al. 2022). Phytophthora species are currently classified into twelve major phylogenetic clades representing a highly versatile group of plant pathogens and saprophytes (Jung et al. 2017; Brasier et al. 2022). The genus includes several important plant pathogenic species that cause economic losses of cash crops in both agriculture and nursery settings. When introduced into natural ecosystems, Phytophthoras may become high-risk pathogens and cause severe damage. For instance, P. cinnamomi is listed as one of the 100 worst invasive alien species (Lowe et al. 2000) and is found in nurseries, parks, gardens, and forests around the globe. In New Zealand, P. agathidicida causes a lethal root rot on Kauri (Agathis australis), which is one of the world’s largest and longest-living conifer species (Bradshaw et al. 2020). Sudden Oak Death in the Western United States (Rizzo et al. 2002) and Sudden Larch Death in Great Britain (Brasier and Webber 2010; Harris and Webber 2016) resulting from the accidental introduction of P. ramorum possibly via plant trade challenge the local commercial timber production. The ever-increasing reports of Phytophthora-associated tree declines and emerging diseases indicate that Phytophthora species will continue to threaten biodiversity and the sustainability of forest ecosystems worldwide (Jung et al. 2017; Brasier et al. 2022).
The devastating impact of some Phytophthora species in natural ecosystems resulted in several large-scale surveys to assess their occurrence. In this context, leaf baiting is commonly used to survey risk areas for the presence of quarantine Phytophthora species (Sutton et al. 2009; O'Hanlon et al. 2018). Indeed, water plays a crucial role in facilitating the dispersal of asexual and short-lived zoospores, which serve as the primary propagules responsible for both dispersion and infection in oomycetes. At least 85 Phytophthora species from most clades are found to disperse in aquatic environments disassociated from their plant host, and 41 species from five clades have adapted to a primarily aquatic lifestyle (Brasier et al. 2022). These species are considered mostly benign or weak pathogens and derive their energy and nutrition from decaying organic matter (Marano et al. 2016; Brasier et al. 2022). Phytophthora clade 6, which complete their life cycle in water (Hansen et al. 2012; Marano et al. 2016), dominate the aquatic environment, but their ecological function is still unclear. Monitoring efforts have led to the discovery of many Phytophthora species in new habitats and the description of new species and previously uncharacterized hybrids (e.g., Scott et al. 2013; Hansen 2015; Jung et al. 2017; Van Poucke et al. 2021).
In Switzerland, the first Phytophthora species officially reported was P. infestans, the causal agent of potato late blight, in 1845 (Dufour 1889). Until 1995, only 15 species had been identified, most of which in orchards and agricultural crops: P. cactorum (first report 1904), P. erytroseptica (1912), P. × cambivora (1942), P. porri (1964), P. syringae (1975), P. megasperma (1976), P. cryptogea (1976), P. citricola (1976), P. drechsleri (1977), P. nicotianae (1980), P. fragariae var. rubi (1980), P. fragariae (1981), P. cinnamomi (1981), and P. citrophthora (1981) (Bolay and Schwinn 1986). In 2003, P. ramorum was officially detected on Viburnum × bodnantense in an ornamental nursery (Heiniger and Stadler 2003). As a result, all nurseries that import or produce putative host plants of this quarantine organism are inspected annually (Prospero et al. 2013). These inspections resulted in the detection of P. ramorum in several nurseries, mostly on imported nursery stocks, and in a few cases on outplanted host plants in urban areas (Prospero et al. 2013). These findings also led to surveys in forests, rivers, and streams, which made it possible to gather valuable information on the distribution of Phytophthora species in the country. Here, we present the results of a nationwide Phytophthora monitoring that investigates Phytophthora community composition in Swiss watercourses. Given that in Switzerland, all nurseries tested positive for P. ramorum, and most human infrastructure and activity are concentrated in the Plateau region (i.e., the densely populated area between the Jura Mountains and the Swiss Alps stretching from Geneva to St. Gallen); we focused on watercourses flowing through this area to increase chances of also detecting exotic Phytophthora species.
Materials and methods
Sampled watercourses
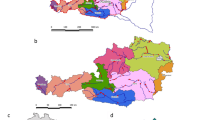
From 2012 to 2016, a total of 32 watercourses (rivers, streams, and creeks) flowing through the highly urbanized Swiss plateau were sampled (Fig. 1, Table 1). These included the three major Swiss rivers Aare (tributary of the Rhine, rises and ends in Switzerland; drainage area of 17779 km2), Rhine (discharging into the North Sea; drainage area of 36472 km2 in Switzerland), and Rhone (discharging into the Mediterranean Sea; drainage area of 8000 km2 in Switzerland), whose sources are in the Swiss Alps, as well as minor streams (Supplementary Figure 1). Watercourse complexity and size were characterized using the Strahler stream classification system (Strahler 1957). According to this system, watercourses of the first order are the outermost tributaries. If two watercourses of the same order merge, the resulting watercourse is given a number that is one higher. If two watercourses with different orders merge, the resulting watercourse is given the higher of the two numbers. The sampled watercourses resulted in a Strahler order from three to nine (https://t.ly/OGJHQ; Table 1). The Person’s correlation coefficient and the corresponding p-value were calculated on https://www.socscistatistics.com/tests/pearson. Each watercourse was also attributed to one of the following three river basins: Aare, Rhine, and Rhone (Supplementary Figure 1 and Supplementary Table 1).
The spatial arrangement of 52 Phytophthora baiting sites, represented by white symbols, spans across 32 watercourses examined for the presence of Phytophthora species in the densely populated Swiss Plateau. Additional details about the baiting sites can be found in Supplementary Figure 1 and Supplementary Table 1
Phytophthora baiting
For Phytophthora baiting, a protocol using rhododendron (Rhododendron catawbiense) leaves as baits was adopted (Oak et al. 2008). The leaves were at least 1 year old and featured a well-developed cuticula without signs of injury or fungal infection. Sampling sites were chosen in sectors of a watercourse with constantly flowing water and adjacent riparian vegetation. For the river Rhone, the sampling site was located close to the river estuary into a lake (Fig. 1).
At each site (52 sites in total; Supplementary Table 1), at least four leaves (individually or in pairs per bait net) were placed on the water surface in a quiet spot between August and October for every year between 2012 and 2016. To allow them to float, the nets were tied to adjacent riparian vegetation with a nylon thread. After approximately 1 week (6–8 days), the nets were retrieved from the water and immediately brought to the laboratory for further analysis.
Phytophthora isolation
All rhododendron leaves were rinsed with demineralised water, dried with paper towels, and subsequently inspected for signs of infection, such as brown and/or transparent lesions on the surface. Such areas were cut into pieces of approximately 3 × 3 cm in size. If there was no sign of infection, parts of the leaf edges and the middle vein were taken. The pieces were then surface sterilized for 1 min in sodium hypochlorite solution (0.5% active chlorine), rinsed twice for 1 min in sterile water, and dried with paper towels. The surface sterilized pieces were cut into six small fragments of approximately 5 × 5 mm and placed on a selective CARP medium containing 17 g cornmeal agar, 10 mg pimaricin, 200 mg ampicillin, 10 mg rifampicin, 15 mg benomyl, and 25 mg hymexazol per 1 L of distilled water (Hansen and Hamm 1996). The CARP plates were incubated at room temperature (20 °C) and checked after 24 h under a microscope. This allowed differentiation between fast- and sparsely-growing mycelium, which is typical for some species of the genus Pythium, and slow- and densely growing Phytophthora mycelium. From the colonies with the latter pattern, an agar plug was transferred to Petri dishes containing Potato Dextrose Agar (39 g/L PDA; Difco, Voight Global Distribution, Lawrence, MD, USA) and incubated at room temperature.
Phytophthora identification
From the putative Phytophthora cultures, an agar plug was either transferred to a Petri dish containing 15 ml V8 liquid medium or directly used for DNA extraction. The V8 medium was prepared by mixing 330 ml of V8 juice with 5 g of calcium carbonate in a sterile flask, stirring for 15–20 min. The mixture was transferred to 50-ml Falcon tubes and centrifuged at 6500 rpm for 5 min to separate solids from liquids. Distilled water was added to the filtered supernatant to reach 600 ml. A total of 200 ml V8 concentrate per 1 L of distilled water is used for the final medium (Miller 1955). After approximately 1 week, the growing mycelium in the V8 liquid medium was harvested using vacuum filtration. About 10 mg of fresh mycelium was put into a sterile Eppendorf tube. DNA extraction was performed using Qiagen DNeasy 96 Plant Kit following the manufacturer’s instructions. From the agar plug, DNA was extracted using LGC Genomics reagents on Kingfisher 96 Flex (Thermofisher) according to the manufacturer’s protocol. DNA concentrations were measured with an Eppendorf Bio Photometer using 54 µl of distilled H2O and 6 µl of pure DNA. Before PCR, DNA samples were tenfold diluted to final concentrations of 1–5 µg/ml. The region spanning the internal transcribed spacer (ITS1-5.81-ITS2) of the ribosomal DNA was PCR amplified and sequenced using the primers ITS6 (5′-GAAGGTGAAGTCGTAACAAGG-3′; Cooke et al. 2000) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′; White et al. 1990). The PCR mix consisted of 10.4 µl distilled and sterile H2O, 7.6 µl of Jump Start Taq polymerase (Sigma Aldrich), 1 µl Primer-Mix ITS6 and ITS4 (12.5. pmol/µl) and 1 µl of tenfold diluted DNA. The PCR was run on Veriti 96 well thermocyclers (Applied Biosystems) with initial denaturation at 95 °C for 2 min, 35 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min, and a final extension of 10 min at 72 °C. Before sequencing, the PCR products were purified using 2 µl of ExoProStar 1-Step (GE Healthcare). For this, 5 µl of the PCR product was incubated for 15 min at 37 °C and for another 15 min at 80 °C.
Cycle sequencing was conducted using 0.75 µl of distilled and sterile H2O, 0.75 µl of Bigdye Sequencing Buffer (5 ×) (Applied Biosystems), 1.5 µl Ready Reaction Premix (2.5 ×) (Applied Biosystems), 1.5 µl of primers ITS6 or ITS4 (3.2 pmol/µl), respectively, and 3 µl of template DNA (5ng/µl). The sequencing reaction was run on Veriti 96 well thermocyclers with 25 cycles consisting of 10 s at 96 °C, 5 s at 50 °C, and 1 min at 60 °C. The cycle sequencing product was cleaned using an XTerminator Kit (Applied Biosystems) and sequenced in both directions on an ABI prism 3130 Genetic Analyser. Sequences were aligned and edited using CLC Main Workbench 7 (www.clcbio.com). For species assignment, the edited sequences were blasted against sequences in the databases Phytophthora–ID.org (http://phytophthoradb.org), BOLD (http://www.boldsystems.org), and NCBI (http://blast.ncbi.nlm.nih.gov). Two sequences were considered to belong to the same species if they showed at least 99% similarity. To confirm species assignment, ITS sequences were analyzed using the NGphylogeny.fr webservice with the PhyML+SMS workflow (Lemoine et al. 2019). For phylogenetic comparisons, representative sequences for each species from our dataset were selected and analyzed along with reference sequences available in NCBI (https://www.ncbi.nlm.nih.gov/) for each of the five detected clades. The phylogeny is presented in Supplementary Fig. 2.
Results
Overall Phytophthora prevalence and diversity
A total of 241 Phytophthora isolates were obtained from the 32 watercourses sampled, with one (seven watercourses) to 42 (one watercourse) isolates found per watercourse (Table 1). The highest Phytophthora prevalence was observed in the rivers Reuss (42 isolates) and Rhine (41 isolates). Noteworthy, in the main river Rhone, which was sampled at one site in proximity to its estuary into the lake of Geneva, only one isolate of Phytophthora was obtained. Sequencing of the ITS region showed that the isolates belonged to 11 different Phytophthora species from five clades (Table 1). Clade 6 was the most represented with five species (P. lacustris, P. gonapodyides, P. riparia, P. chlamidospora, and P. bilorbang), followed by clade 9 (P. hydropathica, P. polonica) and clade 2 (P. plurivora, P. citrophthora) with two species each and clade 8 (P. cryptogea) and clade 10 (P. gallica) with one species each. Overall, P. lacustris was the most common species, accounting for 75.5% of the isolates found, and was present in 30 of the 32 watercourses (93.8%) sampled (Table 1). The second most abundant species was P. plurivora (12.4% of the isolates, 11 watercourses), followed by P. gonapodyides (5.8% of the isolates, nine watercourses). The remaining species were each restricted to one or two watercourses, ranging from one to four isolates each. The number of Phytophthora species found per watercourse ranged from one to five (mean of 1.8; Table 1) and was not significantly influenced by the complexity and size of the watercourse as measured by the Strahler order number (Person’s correlation coefficient r = 0.2523, p = 0.164).
Phytophthora diversity in river basins
In a single basin, three (Rhone) to eight (Aare) Phytophthora species were detected (Fig. 2). Three species, P. lacustris, P. plurivora, and P. gonapodyides, were found in all three basins, with P. lacustris being the predominant species everywhere, accounting for 60% (Rhone) to 80% (Aare) of the isolates. Phytophthora plurivora showed a similar prevalence in the basins Rhine and Rhone (17.2% and 20% of the isolates, respectively), but was considerably less frequent in the Aare basin (3.5%). On the other hand, P. gonapodyides was more frequent in the Rhone basin (20%) than in the other two (4.7% Aare and 6% Rhine). Private species (i.e., species specific to a single basin) were observed in the Aare (P. hydropathica, P. riparia, P. chlamydospora, P. citrophthora, P. bilorbang) and the Rhine (P. cryptogea, P. polonica, P. gallica), but not in the Rhone basin (Fig. 2). Overall, the prevalence of private species was low, ranging from 0.7 (P. gallica) to 4.7% (P. hydropathica) of all isolates.
The proportional representation of Phytophthora diversity in each basin. Phytophthora species were identified in the three sampled river basins within the Swiss Plateau (N denotes the number of watercourses analyzed, as specified in Supplementary Table 1). The prevalence of these species, expressed as a percentage of isolates, is provided in parentheses
Discussion
This study is the result of an intensive Phytophthora monitoring that was conducted between 2012 and 2016 in watercourses flowing through the Swiss Plateau. Using a baiting approach, we isolated Phytophthora in all watercourses sampled. Phytophthora diversity ranged from one to five species per watercourse, which is in line with the values reported in Austria and the Czech Republic (Corcobado et al. 2023) and in Australia (Hüberli et al. 2013). Since complex watercourses consisting of multiple branches can collect water from different regions, one may expect higher Phytophthora diversity in watercourses of higher Strahler orders. However, in our surveys, we did not observe such a correlation. For example, in the main European river Rhone, before its estuary into Lake Geneva (mean annual water discharge 1935–2019: 182 m3/s), only one Phytophthora isolate was recovered. Hence, other factors may play a role in the Phytophthora diversity in watercourses, which may include water temperature and chemistry, and the environment drained (e.g., urban areas, forests, and agricultural land) (Redondo et al. 2018; Riolo et al. 2020).
The 241 Phytophthora isolates detected belonged to 11 different species from five different major clades (Cooke et al. 2000; Yang et al. 2017). Phytophthora clade 6 dominated in the watercourses of the Swiss Plateau, confirming results of previous studies from Central Europe and other continents (e.g., Corcobado et al. 2023; Jung et al. 2011; Reeser et al. 2011; Nagel et al. 2015). The most frequent species was P. lacustris, a ubiquitous species in riparian ecosystems throughout Europe and North America (Nechwatal and Mendgen 2006; Reeser et al. 2011; Nechwatal et al. 2013). The exact ecological function of most taxa within clade 6 is still unclear (Brasier et al. 2003). The adaptation of the nutritional strategies of most species towards a saprophytic lifestyle, lacking an obligate biotrophic phase, is thought to explain, at least partially, their dominance in water (Aram and Rizzo 2018). For instance, the facultative pathogens P. gonapodyides and P. chlamydospora although capable of causing disease symptoms (Hansen 2015; Ruffner et al. 2019) may have a competitive advantage over other colonizers with an obligate biotrophic phase. Sexual sterility and tolerance to high temperatures have also been suggested to represent an adaptation of clade 6 species to riparian conditions (Brasier et al. 2003). Although natural hybridization among clade 6 Phytophthora species seems to be a common phenomenon (e.g., in Western Australia, Burgess 2015; South Africa, Nagel et al. 2013; Central Europe, Corcobado et al. 2023), no hybrids were found in our study. Regarding the non-clade 6 Phytophthora species detected in the Swiss watercourses, all of them were already reported to occur in streams and rivers across Europe (e.g., Jung et al. 2019; Christova 2022; Corcobado et al. 2023). To mention, there is a relatively high prevalence of P. plurivora (12.4% of the isolates, present in 11 watercourses), a species commonly found in Europe where it is frequently associated with declining trees, especially European beech (Fagus sylvatica) (e.g., Ruffner et al. 2019; Corcobado et al. 2020; Jankowiak et al. 2023).
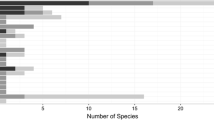
In comparison to the findings in 1996 and 2013, where 15 and nine Phytophthora species were respectively reported in Switzerland (Bolay and Schwinn 1996; Scott et al. 2013), our recent monitoring in Swiss watercourses identified a total of 11 species. Of these, six were reported for the first time in the country (P. bilorbang, P. gallica, P. hydropathica, P. lacustris, P. polonica, P. riparia). Phytophthora bilorbang (clade 6) was first described in 2012 in Western Australia as a pathogen of Rubus anglocandicans (Aghighi et al. 2012). Later, it was also isolated in Southern Italy from the rhizosphere and from declining plants of the Mediterranean maquis, as well as from olive trees (Olea europaea) with symptoms of defoliation, wilting, and root rot (Scanu et al. 2015; Santilli et al. 2020) and in Central Europe from streams and rivers (Corcobado et al. 2023). Phytophthora gallica (clade 10) was previously found in the rhizosphere of a declining oak in Northeastern France and in the rhizosphere of common reed (Phragmites australis) in Southwestern Germany (Jung and Nechwatal 2008). Phytophthora hydropathica (clade 9) was first described by Hong et al. (2010) from irrigation water in ornamental plant nurseries in the Eastern United States. Interestingly, both soilborne species P. gallica and P. hydropathica were also detected in watercourses in Austria and the Czech Republic (Corcobado et al. 2023), which, together with our study, confirms their occurrence in aquatic environments in Central Europe. The clade 8 species P. polonica has so far been reported only in Europe, mainly in association with declining alders (Alnus spp.) (Belbahri et al. 2006, Corcobado et al. 2023; Matsiakh et al. 2023; Tkaczyk et al. 2023). Finally, after being first identified in streams in Alaska and Oregon (Hansen et al. 2012), P. riparia (clade 6) has also been found in rivers and streams of other US states (Stamler 2016; Bily et al. 2022) and Central Europe (Corcobado et al. 2023), as well as in plant nurseries in California (Rooney-Latham et al. 2019).
To date, a total of 30 Phytophthora species have been officially confirmed in Switzerland, twice as many as were reported in 1996 (Fig. 3). In addition to species that are most likely native to Europe (e.g., P. cactorum, P. gonapodyides, P. lacustris, P. polonica), exotic species are also present, many of which have a high pathogenic potential (in particular, P. × cambivora, P. cinnamomi, P. × alni, P. infestans, P. ramorum). As revealed in other studies, species are not evenly distributed across environments but show typical occurrence patterns (Fig. 3). Hence, to obtain an accurate picture of the diversity and distribution of Phytophthora species in a given region, it is necessary to sample different types of environments. Our surveys confirm a strong preference for clade 6 species for aquatic environments, in which P. hydropathica, P. polonica (clade 9), and P. gallica (clade 10) have also been found so far. Invasive pathogenic species are restricted to tree nurseries (P. multivora and P. ramorum) and/or forests (P. × alni, P. × cambivora, P. cinnamomi). None of them could be detected in watercourses, possibly reflecting their effective absence due to successful eradication measures (e.g., P. ramorum) or their still relatively low prevalence in terrestrial environments (i.e., not enough inoculum to reach watercourses). On the other hand, such species may be less competitive in the aquatic environment. For example, P. cinnamomi, which is the main causal agent of the ink disease of sweet chestnut (Castanea sativa) in Southern Switzerland (Prospero et al. 2023), could not be detected in the Ticino River, into which water from infected chestnut stands flows (unpublished data). A similar situation was, for example, reported in Sardinia (Italy) in watercourses draining declining cork oak (Quercus suber) stands in which P. cinnamomi was the most common species in the soil (Seddaiu et al. 2020). Stream baiting has been useful to survey invasive Phytophthora species such as P. ramorum, but it might not be the method of choice to monitor other Phytophthora species, in particular obligate biotrophs.
Phytophthora species detected in Switzerland up to 2023. Phytophthora species were grouped into ITS Clades following Yang et al. (2017)1. Historial data2: Bolay & Schwinn (1996). Watercourse3: This study. Forests4 (includes urban trees): Ruffner et al. (2019), Mizeriene et al. (2020), and Prospero et al. (2023). Nurseries5: Prospero et al. (2013). P. plurivora6 this species identified as P. citricola in Bolay and Schwinn (1996)
Data availability
Sequences of the Phytophthora cultures detected in this study are available from the corresponding author upon request.
References
Aghighi S, Hardy GESJ, Scott JK, Burgess TI (2012) Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. Eur J Plant Pathol 133:841–855
Aram K, Rizzo DM (2018) Distinct trophic specializations affect how Phytophthora ramorum and clade 6 Phytophthora spp colonize and persist on Umbellularia californica leaves in streams. Phytopathology 108:858–869
Belbahri L, Moralejo E, Calmin G, Oszako T, García JA, Descals E, Lefort F (2006) Phytophthora polonica, a new species isolated from declining Alnus glutinosa stands in Poland. FEMS Microbiol Lett 261:165–174
Bily D, Nikolaeva E, Olson T, Kang S (2022) Phytophthora spp. associated with appalachian oak forests and waterways in Pennsylvania, with P. abietivora as a pathogen of five native woody plant species. Plant Dis 106:1143–1156
Bolay A, Schwinn FJ (1996) Phytophthora species in Switzerland. Mycologia Helvetica 8:21–71
Bradshaw RE, Bellgard SE, Black A, Burns BR, Gerth ML, McDougal RL, Scott PM, Waipara NW, Weir BS, Williams NM, Winkworth RC, Ashcroft T, Bradley EL, Dijkwel PP, Guo Y, Lacey RF, Mesarich CH, Panda F, Horner IJ (2020) Phytophthora agathidicida: research progress, cultural perspectives and knowledge gaps in the control and management of kauri dieback in New Zealand. Plant Pathol 69:3–16
Brasier CM, Webber JF (2010) Sudden larch death. Nature 466:824–825
Brasier CM, Cooke DEL, Duncan JM, Hansen EM (2003) Multiple new phenotypic taxa from trees and riparian ecosystems in Phytophthora gonapodyides-P. megasperma ITS Clade 6, which tend to be high-temperature tolerant and either inbreeding or sterile. Mycol Res 107:277–290
Brasier C, Scanu B, Cooke D, Jung T (2022) Phytophthora: an ancient, historic, biologically and structurally cohesive and evolutionarily successful generic concept in need of preservation. IMA Fungus 13:12
Burgess TI (2015) Molecular characterization of natural hybrids formed between five related indigenous clade 6 Phytophthora species. PLOS ONE 10:e0134225
Christova PK (2022) Detection of Phytophthora gallica in Bulgaria and co-existence with other Phytophthora species in a small river. J Plant Dis Prot 129:1377–1387
Cooke DEL, Drenth A, Duncan JM, Wagels G, Brasier CM (2000) A molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet Biol 30:17–32
Corcobado T, Cech TL, Brandstetter M, Daxer A, Hüttler C, Kudláček T, Horta Jung M, Jung T (2020) Decline of European beech in Austria: involvement of Phytophthora spp. and contributing biotic and abiotic factors. Forests 11:895
Corcobado T, Cech TL, Daxer A, Ďatková H, Janoušek J, Patra S, Jahn D, Hüttler C, Milenković I, Tomšovský M, Horta Jung M, Jung T (2023) Phytophthora, Nothophytophthora and Halophytophthora diversity in rivers, streams and riparian alder ecosystems of Central Europe. Mycol Prog 22:50
Dufour J (1889) Note sur l’action du sulfate de cuivre sur la germination de quelques champignons. Landw Jb Schweiz 3:97–104
Hansen EM (2015) Phytophthora species emerging as pathogens of forest trees. Curr Forestry Rep 1:16–24
Hansen EM, Hamm PB (1996) Survival of Phytophthora lateralis in infected roots of Port Orford cedar. Plant Dis 80:1075–1078
Hansen EM, Reeser PW, Sutton W (2012) Phytophthora borealis and Phytophthora riparia, new species in Phytophthora ITS Clade 6. Mycologia 104:1133–1142
Harris AR, Webber JF (2016) Sporulation potential, symptom expression and detection of Phytophthora ramorum on larch needles and other foliar hosts. Plant Pathol 65:1441–1451
Heiniger U, Stadler B (2003) Gefährliche Quarantänekrankheit gefunden. Der Gartenbau 51(52):10–12
Hong CX, Gallegly ME, Richardson PA, Kong P, Moorman GW, Lea-Cox JD, Ross DS (2010) Phytophthora hydropathica, a new pathogen identified from irrigation water, Rhododendron catawbiense and Kalmia latifolia. Plant Pathol 59:913–921
Hüberli D, Hardy GESJ, White D, Williams N, Burgess TI (2013) Fishing for Phytophthora from Western Australia’s waterways: a distribution and diversity survey. Australasian Plant Pathol 42:251–260
Jankowiak R, Stępniewska H, Bilański P, Kolařík M, Taerum SJ (2023) Phytophthora species cause sudden and severe decline of naturally regenerated European beech (Fagus sylvatica) seedlings. Plant Pathol 72:774–785
Jung T, Nechwatal J (2008) Phytophthora gallica sp nov., a new species from rhizosphere soil of declining oak and reed stands in France and Germany. Mycol Res 112:1195–1205
Jung T, Stukely MJC, Hardy GESJ, White D, Paap T, Dunstan WA, Burgess TI (2011) Multiple new Phytophthora species from ITS Clade 6 associated with natural ecosystems in Australia: evolutionary and ecological implications. Persoonia 26:13–39
Jung T, Horta Jung M, Cacciola SO, Cech T, Bakonyi J, Seress D, Mosca S, Schena L, Seddaiu S, Pane A, di San Magnano, Lio G, Maia C, Cravador A, Franceschini A, Scanu B (2017) Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 8:219–244
Jung T, La Spada F, Pane A, Aloi F, Evoli M, Horta Jung M, Scanu B, Faedda R, Rizza C, Puglisi I, di San Magnano, Lio G, Schena L, Cacciola SO (2019) Diversity and distribution of Phytophthora species in protected natural areas in sicily. Forests 10:259
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species a selection from the global invasive species database. Published by the Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), pp 12
Marano AV, Jesus AL, De Souza JI, Jerônimo GH, Gonçalves DR, Boro MC, Rocha SCO, Pires-Zottarelli CLA (2016) Ecological roles of saprotrophic Peronosporales (Oomycetes, Straminipila) in natural environments. Fun Ecol 19:77–88
Matsiakh I, López-García N, Kacprzyk M, Cleary M (2023) Susceptibility of silver birch and black alder to several Phytophthora species isolated from soils in declining broadleaf forests in western Ukraine. For Path 53:e12817
Miller PM (1955) V8 juice agar as a general-purpose medium for fungi and bacteria. Phytopathology 45:461–462
Mizeriene G, Cerny K, Zyka V, Bakonyi J, Nagy ZA, Oliva J, Redondo MA, Corcobado T, Prospero S (2020) Patterns of genetic diversification in the invasive hybrid plant pathogen Phytophthora × alni and its parental species P. uniformis. Phytopathology 110:1959–1969
Nagel JH, Gryzenhout M, Slippers B, Wingfield MJ, Hardy GESTJ, Stukely MJC, Burgess TI (2013) Characterization of Phytophthora hybrids from ITS clade 6 associated with riparian ecosystems in South Africa and Australia. Fun Biol 117:329–347
Nagel JH, Slippers B, Wingfield MJ, Gryzenhout M (2015) Multiple Phytophthora species associated with a single riparian ecosystem in South Africa. Mycologia 107:915–925
Nechwatal J, Mendgen K (2006) Widespread detection of Phytophthora taxon salixsoil in the littoral zone of lake constance, Germany. Eur J Plant Pathol 114:261–264
Nechwatal J, Bakonyi J, Cacciola SO, Cooke DEL, Jung T, Nagy ZA, Vannini A, Vettraino AM, Brasier CM (2013) The morphology, behaviour and molecular phylogeny of Phytophthora taxon Salixsoil and its redesignation as Phytophthora lacustris sp. nov. Plant Pathol 62:355–369
O’Hanlon R, Choiseul J, Brennan JM, Grogan H (2018) Assessment of the eradication measures applied to Phytophthora ramorum in Irish Larix kaempferi forests. For Pathol 48:1
Oak SW, Elledge AH, Yockey EK, Smith WD, Tkacz BM (2008) Phytophthora ramorum early detection surveys for forests in the United States, 2003–2006. In: Frankel SJ, Kliejunas JT, Palmieri KM (eds) Proceedings of the sudden oak death third science symposium. Gen. Tech. Rep. PSW-GTR-214. U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station, Albany, pp 413–416
Prospero S, Vercauteren A, Heungens K, Belbahri L, Rigling D (2013) Phytophthora diversity and the population structure of Phytophthora ramorum in Swiss ornamental nurseries. Plant Pathol 62:1063–1071
Prospero S, Heinz M, Augustiny E, Chen YY, Engelbrecht J, Fonti M, Hoste A, Ruffner B, Sigrist R, van den Berg N, Fonti P (2023) Distribution, causal agents, and infection dynamic of emerging ink disease of sweet chestnut in southern Switzerland. Environ Microbiol 25:2250–2265
Redondo MA, Boberg J, Stenlid J, Oliva J (2018) Functional traits associated with the establishment of introduced Phytophthora spp. in Swedish forests. J Appl Ecol 55:1538–1552
Reeser PW, Sutton W, Hansen EM, Remigi P, Adams GC (2011) Phytophthora species in forest streams in Oregon and Alaska. Mycologia 103:22–35
Riolo M, Aloi F, La Spada F, Sciandrello S, Moricca S, Santilli E, Pane A, Cacciola SA (2020) Diversity of Phytophthora communities across different types of Mediterranean vegetation in a nature reserve area. Forests 11:863
Rizzo DM, Garbelotto M, Davidson JM, Slaughter GW, Koike ST (2002) Phytophthora ramorum as the cause of extensive mortality of Quercus spp. and Lithocarpus densiflorus in California. Plant Dis 86:205–214
Rooney-Latham S, Blomquist CL, Kosta KL, Gou YY, Woods PW (2019) Phytophthora species are common on nursery stock grown for restoration and revegetation purposes in California. Plant Dis 103:448–455
Ruffner B, Rigling D, Schoebel CN (2019) Multispecies Phytophthora disease patterns in declining beech stands. For Path 49:e12514
Santilli E, Riolo M, La Spada F, Pane A, Cacciola SO (2020) First report of root rot caused by Phytophthora bilorbang on Olea europaea in Italy. Plants 9:826
Scanu B, Linaldeddu BT, Deidda A, Jung T (2015) Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 10:e0143234
Scott P, Burgess T, Hardy G (2013) Globalization & Phytophthora. In: Phytophthora: A Global Perspective. Ed. by Lamour, K. Wallingford, UK: CABI: 239–232
Seddaiu S, Brandano A, Ruiu PA, Sechi C, Scanu B (2020) An overview of Phytophthora species inhabiting declining Quercus suber stands in Sardinia (Italy). Forests 11:971
Stamler RA, Sanogo S, Goldberg NP, Randall JJ (2016) Phytophthora species in rivers and streams of the Southwestern United States. Appl Environ Microbiol 82:4696–4704
Strahler AN (1957) Quantitative analysis of watershed geomorphology. Trans Am Geophys Union 38:913–920
Sutton W, Hansen EM, Reeser PW, Kanaskie A (2009) Stream monitoring for detection of Phytophthora ramorum in Oregon Tanoak Forests. Plant Dis 93:1182–1186
Tkaczyk M, Sikora K, Galko J, Kunca A (2023) Occurrence of Phytophthora species in riparian stands of black alder (Alnus glutinosa) in Slovakia. For Path 53:e12800
Van Poucke K, Haegeman A, Goedefroit T, Focquet F, Leus L, Horta Jung M, Nave C, Redondo MA, Husson C, Kostov K, Lyubenova A, Christova P, Chandelier A, Slavov S, de Cock A, Bonants P, Werres S, Oliva Palau J, Marçais B, Jung T, Stenlid J, Ruttink T, Heungens K (2021) Unravelling hybridization in Phytophthora using phylogenomics and genome size estimation. IMA Fungus 12:16
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Innis, M. A., et al. (Ed.). PCR Protocols: a Guide to Methods and Applications. Xviii+482p. Academic Press, Inc.: San Diego, California, USA; London, England, UK. Illus:315–322
Yang X, Tyler BM, Hong C (2017) An expanded phylogeny for the genus Phytophthora. IMA Fungus 8:355–384
Acknowledgements
We would like to thank Esther Jung, Hélène Blauenstein, Walter Jungen, and Hanna Vydrzel for much appreciated help in the field and in the laboratory and Tanay Bose for valuable comments and suggestions on the first version of this manuscript. We are grateful to Nicolò Sasso for preparing Fig. 1.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL. The Phytophthora monitoring 2012–2016 was financially supported by the Swiss Federal Office for the Environment (FOEN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Section Editor: Tanay Bose
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical collection - Since de Bary: Progress in Phytophthora research.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schoebel, C.N., Prospero, S., Rigling, D. et al. Fishing for Phytophthora in watercourses of the highly urbanized Swiss Plateau. Mycol Progress 23, 17 (2024). https://doi.org/10.1007/s11557-024-01951-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-024-01951-7