Abstract
Based on phylogenetic analyses of the ITS, nuclear large subunit rRNA, mitochondrial small subunit rRNA, and MCM7 genes, species previously treated as Pannaria hispidula and P. isabellina are shown to represent two new Pannariaceae genera, Hispidopannaria and Phormospsora. Each genus forms monophyletic clades, both in multilocus phylogeny and in single gene phylogenies. In the multilocus phylogeny, both genera together formed a monophyletic clade as a sister group to the genus Pannaria, whereas this monophyly was not maintained in single gene phylogenies. Hispidopannaria differs from Pannaria in having large, geotropically arranged, hispid squamules, IKI+ internal ascus structures, and perispores with irregular pulvinate verrucae and apical extensions. The southern South American, TLC-negative species H. hispidula is generitype and is concentrated to trunks in the evergreen Nothofagus forests of south-central Chile. Psoroma dasycladum, a similar endemic species from the Juan Fernández Archipelago, is also transferred to Hispidopannaria. Phormopsora is monospecific and is the only member of Pannariaceae which contains norstictic and connorstictic acids. Its thallus of large, branched squamules with large, foliose cephalodia and its bullate perispores with long-apiculate apical extensions also separate it from Pannaria. Its species, Phormopsora isabellina, has a similar distribution as H. hispidula on the South American mainland, but is more widespread. The position of these two small genera as a sister group to the large and diverse genus Pannaria, indicates a long period of slow evolutionary rate, with the island endemic Hispidopannaria dasyclada as an exception. Reproductive isolation and photobiont specialization are partly suggested to explain their slow evolution and lack of surviving speciation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of accepted genera in Pannariaceae has increased dramatically in recent years from 16 (incl. two critical genera) in the review by Jørgensen (2006), to 30 accepted ones presented by Ekman et al. (2014). Since then, the generitype of the genus Kroswia has been transferred to Fuscopannaria (Magain and Sérusiaux 2015), Degeliella has been replaced by Psoromaria (Jørgensen and Andersen 2015), and the new genera Gibbosporina and Rockefellera have been described (Elvebakk et al. 2016; Lendemer et al. 2017). In addition to molecular support, the characters most often used to characterize genera in the review by Ekman et al. (2014) are hymenium amyloidity, ascus amyloidity, secondary chemistry, excipulum characters, and gross thallus morphology.
Several of these genera are small, and six of them are monospecific. The latter include the southern South American genus Joergensenia, the North American Rockefellera, and the Palaeotropical Leightoniella, which are well supported in terms of molecular phylogeny and morphology/anatomy/chemistry (Passo et al. 2008; Lendemer et al. 2017; Weerakoon et al. 2018). Nevesia and Steineropsis were published with molecular and anatomical support, but have not yet been found with apothecia/mature apothecia (Ekman et al. 2014; Spribille and Muggia 2013). Psoromidium was recently presented as a monophyletic and monotypic genus, but with modest support (Jørgensen and Andersen 2015). In the same study, it was shown that the name Psoromaria has priority over Degeliella, but its two species do not form a monophyletic clade. The monophyly of the small genus Austrella was also recently distorted by the addition of a third species (Fryday et al. 2017). Three small genera of two species each, Nebularia, Psorophorus, and Xanthopsoroma are apparently well-supported and monophyletic (Elvebakk et al. 2010; Ekman et al. 2014).
Whereas large genera such as Fuscopannaria, Pannaria and Parmeliella have wide distributions, most of the small genera are from the Southern Hemisphere, where Pannariaceae has its highest diversity. The number of Pannariaceae species in New Zealand treated by de Lange et al. (2012, 2018) was 90 and will certainly increase. Large, foliose Pannaria species are still being described (e.g., Elvebakk and Elix 2017), and the knowledge of squamulose species is even less satisfactory, with a number of mostly corticolous species in doubtful positions within the widely circumscribed genera Pannaria and Psoroma. They deviate so much from the generitypes of these genera that the concepts of the latter become too wide and too vaguely defined. This is particularly the case with Pannaria hispidula (Nyl.) Hue and P. isabellina (Vain.) Elvebakk & Bjerke.
Psoroma hispidulum Nyl. was briefly described in a small paper by Nylander (1855) dealing with lichens collected in Peru and Chile by Lechler. Nylander described it as gray to lead gray, hispid, with imbricate squamules on a black hypothallus, and with crenate apothecia with ellipsoid ascospores, 14–16 × 8 μm in size, and with distinct perispores. He only presented it as “corticolous in Chile,” but added the type locality information later (Nylander 1863). Nylander repeated this finding in several papers, also in the one about Fuegian-Patagonian lichens (Nylander 1888), but there is no mentioning of the species from Tierra del Fuego as indicated by Grassi (1950). Hue (1902) treated it as Pannaria hispidula (Nyl.) Hue, and later (Hue 1908) provided an extended description, although he wrote that he could not find mature spores in the apothecia that were sectioned. He cited two Lechler 854 specimens, one in “herb. Mus. paris.,” and one “in meo.” The type collections are now represented by the lectotype in H, lectotypified by Jørgensen (2003), by an additional isolectotype there, one more at PC, one at W, and no less than four isolectotypes at BM.
The species was first treated phylogenetically by Passo et al. (2008), based on ITS and mitochondrial small subunit rRNA genes of a specimen (Passo 112) from Argentina. Here, the species was positioned in a widely defined Pannaria clade, and they treated P. hispidula and P. implexa (Stirt.) Passo, Calvelo & Stenroos as closely related to Pannaria isabellina, also represented with a single specimen (Passo 250) from Argentina. The same sequences were included in trees published by Elvebakk et al. (2010), Ekman et al. (2014), and Fryday et al. (2017), in all cases in unresolved positions as sister groups to various parts of the trees, but not as a sister group to Pannaria s. str. only.
The only species which looks similar, with the same type of hairs and similar, but more compact, ascending squamules, is Psoroma dasyphyllum Zahlbr. It has only been published from its type locality in the Robinson Crusoe Island of the Chilean Juan Fernández Islands (Zahlbruckner 1924) and is included in the present study.
Psoroma isabellinum Vain., the other deviating species to be studied here, was described from a single collection in Chile by Vainio (1899). It was studied by Elvebakk and Bjerke (2005), who reported on the presence of norstictic and stictic acids, unique within Pannariaceae, and “long-tailed” and bullate perispores. A combination of these and other characters were stated to indicate a position in an undescribed genus. However, a description of a new genus for it was then found to be premature without any molecular support.
Thus, the aim of the present study is to study these deviating taxa, characterize them within two new genera by similar characters as usually used to circumscribe Pannariaceae genera, and document their monophyly by more genetic markers than previously used in Pannariaceae studies.
Materials and methods
Taxon sampling and identification
Herbarium materials from B, BG, BM, CHR, H, KASS, NY, PC, S, SGO, TROM, TUR, UPS, UV, and W were studied. A total of 60 samples were examined. In microscope sections, iodine reactions were tested by adding IKI to mounts pretreated with KOH (Orange et al. 2001). Perispore structures were studied in water mounts and restricted to spores liberated from the asci. Thin-layer chromatography of acetone extracts followed standardized procedures and used solvents A and C (Culberson 1972; Orange et al. 2001). Nomenclature of ascospore structures follows Nordin (1997). Several type specimens have been illustrated in the JSTOR Global Plant database at http://plants.jstor.org. The complete URL addresses for the illustrated specimens are not cited here, but can be searched for below their basionym names.
DNA extraction, and sequencing
Sequences of four phylogenetic markers, ITS1-5.8S-ITS2 rRNA (ITS), nuclear large subunit rRNA (LSU), mitochondrial small subunit rRNA (mtSSU), and minichromosome maintenance component 7 (MCM7) genes, of 44 specimens including 2 specimens of Hipidopannaria and 7 specimens of Phormopsora, were determined (Suppl. Table 1). The freeze-dried lichen materials were ground using TissueLyser (Qiagen, Hilden, Germany) after freezing in liquid nitrogen, and genomic DNAs were extracted using Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) or FastDNA spin kit for soil (MP biomedicals, California) according to the manufacturer’s guide. The ITS- LSU domains was amplified using ITS1F and LR5 by the procedures as described in a previous study (Elvebakk et al. 2010). The primers mrSSU1 and mrSSU3R (Zoller et al. 1999) were used for amplification of mtSSU, following the procedures described in a previous study (Park et al. 2018). MCM7 was amplified using the primers, mcm7-709for and mcm7-1348rev (Schmitt et al. 2009). Touch-down PCR amplifications were performed in a T-gradient thermocycler (Biometra, Germany) with the following cycling parameters: 1 min initial denaturation at 95 °C, 6 touchdown cycles of 30 s, denaturation at 95 °C, 50 s. annealing at 60–56 °C at the ramp of 1° per cycle, and 1 min extension at 72 °C, followed by 38 cycles of 45 s denaturation at 94 °C, 50 s annealing at 56 °C, and 1 min extension at 72 °C, and 5 min final extension at 72 °C. The sequences were deposited at the GenBank database and accession numbers are listed in Suppl. Table 1.
Phylogenetic analyses
All the phylogenetic analyses included reference sequences to represent major phylogenetic lineages of Pannariaceae. The sequences are newly published in the present study or have been published previously (Ekman and Jørgensen 2002; Wedin et al. 2007, 2009¸ Passo et al. 2008; Elvebakk et al. 2010, 2016; Ekman et al. 2014; Park et al. 2018). Pannaria implexa (Stirt.) Passo, Stenroos & Calvelo as analyzed by Passo et al. (2008) was redetermined to P. byssoidea Passo & Calvelo by Passo and Calvelo (2011). The present dataset comprises 51 specimens of Pannariaceae with one sample of Parmeliella triptophylla (Ach.) Müll. Arg. as an outgroup (Suppl. Table 1). ITS sequences were available for all the samples, but sequence information of LSU, mtSSU, and MCM7 genes were missing for some of them. Sequence alignments were conducted by the software ClustalX (Larkin et al. 2007) and manually adjusted. Because of size variation and difficult alignment of the ITS1 domain, it was not included in the phylogenetic analyses. Ambiguously aligned sites were excluded from the phylogenetic analyses. Phylogenetic trees were inferred from the combined dataset and each genetic locus by maximum parsimony (MP), maximum likelihood (ML), and Bayesian analyses. MP trees were obtained using the Tree-Bisection-Regrafting (TBR) algorithm of MEGA X (Kumar et al. 2018) with search level 5 in which the initial trees were obtained by the random addition of sequences (1000 replicates). ML trees were constructed using MEGA X based on the GTR + I + G evolutionary model (Lanave et al. 1984), and the search options of best tree topology finding by branch swapping of NNIs and SPRs and random addition of sequences (1000 replicates). The Bayesian tree was searched for by MrBayes ver. 3.2. (Ronquist et al. 2012) with the GTR + I + G model. Two parallel Markov Chain Monte Carlo (MCMC) runs were performed for 1000,000 cycles, each with one cold and three heated chains and the temperature parameter set to 0.1; trees were sampled every 100 generations. A consensus tree was calculated after discarding the first 25% trees as burn-in.
Results
Phylogeny
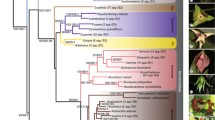
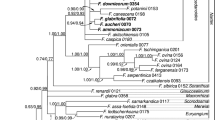
The phylogeny of Pannariaceae based on the combined dataset of ITS, LSU, mtSSU, and MCM7 is shown in Fig. 1. The tree includes 11 available sequences of Pannaria hispidula (labeled as “Hispidopannaria hispidula”) and P. isabellina (labeled as “Phormopsora isabellina”) together with a selection of 12 additional reference sequences of Pannaria and 10 reference sequences of Psoroma. A few sequences of the related genera, Psorophorus, Fuscoderma, Fuscopannaria, Protopannaria, Xanthopsoroma, Gibbosporina, Physma, and Lepidocollema were also included. Monophyly of samples included in P. hispidula was consistently recovered and well supported with high bootstrap values by all phylogenetic methods, ML, MP, and Bayesian, and all phylogenetic markers, ITS, LSU, mtSSU, and MCM7, as well as by the combined dataset. The species formed a clearly separate clade from the other genera of Pannariaceae. Monophyly and a distinct clade formation of Pannaria isabellina was also consistently recovered by all methods and all phylogenetic markers. The two species formed a monophyletic group in the Bayesian tree based on the combined dataset and was a sister group of the genus Pannaria. However, relationships varied when a single phylogenetic marker was used in the analyses (Fig. S1). The monophyletic grouping of both genera assembled within a single clade was maintained with good support when using the MCM7 marker only (Fig. S1d), maintained with poor support when using ITS2 only (Fig. S1a), but distorted when using LSU or mtSSU only (Fig. S1b, c).
Bayesian tree based on combined sequences of ITS, LSU, mtSSU, and MCM7. Black thick branches indicate those that were conserved in both ML and MP trees. Grey thick branches indicate those that were conserved in one of ML or MP trees. Bayesian posterior probabilities (PP ≥ 0.90) and bootstrap values in ML and MP trees (≥ 80%) are indicated above or under the near branches (PP/ML/MP). Asterisks indicate branches that were not supported by high bootstrap values
Phylogenetic positions of other genera and clades such as Xanthopsoroma, the Psoroma tenue clade, and the Pannaria byssoidea clade were also variable when the results in the combined dataset was compared with single phylogenetic markers used separately. The genus Xanthopsoroma is the sister group of a major part of Pannariaceae in the combined dataset and in the tree using mtSSU only, but had different phylogenetic positions when using the other markers separately. The Psoroma tenue clade was closely related to the P. hypnorum clade and Psorophorus in the combined dataset and in phylograms using ITS, mtSSU, MCM7 only, but it was distantly related with these clades in the LSU phylogram. The Pannaria byssoidea clade formed a monophyletic group in a sister group position to the remaining members of the genus Pannaria in the combined dataset and in the phylogram using mtSSU only, but in different positions in the other phylograms. The P. hypnorum, the Fuscoderma, and the Physma clades showed close relationships in all trees, although the relationships among them were variable depending on the markers.
Taxonomy
Hispidopannaria Elvebakk, S.G. Hong & C.H. Park, gen. nov.
MycoBank: MB 837372.
Generitype: Hispidopannaria hispidula (Nyl.) Elvebakk, S.G. Hong & C.H. Park.
Diagnosis: Differs from Pannaria by forming large, geotropically arranged, hispid squamules, by having asci with IKI + blue apical cap-like structures, and ascospores with perispores with irregularly positioned pulvinate verrucae and pulvinate to reniform apical extensions.
Etymology: From Latin “hispidus” (= covered by coarse, rigid hairs), in combination with its relationship to Pannaria.
Description: Thallus of chloromorph 5–30 cm diam., corticolous or saxicolous, large-squamulose. Squamules erect from a more or less distinct, blackish hypothallus, subdichothomously divided, forming a contiguous mat, 3–7 mm tall. Lobes 0.3–0.5 mm wide, 150–400 μm thick. Upper surface grayish when fresh and dry, dark salad-green when fresh and moist, gradually turning chestnut brown in old herbarium specimens, matt, distinctly to occasionally hispid from hyaline erect hairs, 60–100 μm long, 5–10 μm thick, with indistinct septae. Major photobiont Trebouxia. Nostoc cells organized within conspicuous, foliose to fruticose and 1–5 mm large cephalodia, also hispid. Apothecia common, 1–3.5 mm, with crenate thalline excipuli, hymenium IKI+ blue, asci with cap-like, apical IKI+ blue structures. Proper spores ellipsoid, surrounded by smooth perispores, except for apical pulvinate to reniform extensions and scattered pulvinate verrucae. Pycnidia forming verrucae with small bacilliform spermatia.
Chemistry: pannarin or TLC-negative.
Hispidopannaria dasyclada (Zahlbr.) Elvebakk, comb. nov.
MycoBank: MB 837374.
≡ Psoroma dasycladum Zahlbr. in Skottsberg (ed.): Nat. Hist. Juan Fern. Easter Isl. II Bot. 11: 341 (1924) Type: Juan Fernandez Isl.: Masatierra, Felskamm umweit Tres Puntas, 350 m, auf Humuserde, 6 Jan. 1917, C. & I. Skottsberg, UPS!, holotype; S, L732, isotype! http://plants.jstor.org; BM isotype!; SGO isotype!: NY 01304885 isotype! http://plants.jstor.org; W isotype! The isotypes are designated here (Figs. 2 and 3).
Hispidopannaria hispidula. a The specimen Elvebakk 07:309, photographed in the field when moist. The specimen to the left measures 30 cm across. b The specimen Mahu s.n., B 60 0166453. c Ascospores of Hispidopannaria hispidula (above, two Cuvertino 60 spores to the right) and H. dasyclada (below). Scale-bar 15 μm
Description (supplement to Zahlbruckner 1924): Lobe apices often hispid from hyaline erect hairs, 50–100 μm long, 5–10 μm thick, with indistinct septae. Chlorobiont Trebouxia, cells globose to subglobose, 10–20 μm. Cephalodia present, small-foliose to small-fruticose, forming 1–3 mm large rosettes inbetween chlorobiont squamules, hispid and basically similar to the latter, but lobes smaller and 0.1–0.3 mm broad. Perispores verrucose when immature, later with irregular gibbae and scattered pulvinate verrucae. Apical extensions absent, pulvinate or reniform. Asci with IKI + blue internal apical sheath-like structures.
Chemistry: pannarin (major).
Additional specimens studied. CHILE. Arquipiélago Juan Fernández: Isla Robinson Crusoe (“Masatierra”), Portezuelo (Miradór), 500 m, 7 Nov. 1954, G. Kunkel 308c/6 (B); Trail to Damajuana, 400 m, 30 Jan. 1955, G. Kunkel 307/6 & 7 (B).
Hispidopannaria hispidula (Nyl.) Elvebakk, S.G. Hong & C.H. Park, comb. nov.
MycoBank: MB 837375.
≡ Psoroma hispidulum Nyl. in Flora 38: 647 (1855) ≡ Pannaria hispidula (Nyl.) Hue in Bull. Soc. Bot. Fr. 48: 57 (1902). Type: Chile: Arique in alta montana regione Myrtus Lumae, Feb. 1852, W. Lechler H-NYL 30816 lectotype!, lectotypified by Jørgensen (2003: 69), ill. at http://plants.jstor.org.; H-NYL 30815 isolectotype! ill. at http://plants.jstor.org; isolectotype PM!; W isolectotype!; four isolectotypes BM!), isolectotypifications made here (Fig. 2).
Description: Thallus of chloromorph 5–30 cm diam., corticolous, large-squamulose. Squamules geotropically arranged on a large and distinct blackish hypo−/prothallus. In peripheral parts numerous, tiny to 5 mm diam., discretely positioned directly on the hypothallus and more or less adpressed, in central parts of thalli, erect, imbricate, subdichothomously divided, forming a contiguous mat, 3–7 mm tall. Lobes 0.3–0.5 mm wide, 150–250 μm thick. Upper surface grayish when fresh and dry, dark salad-green when fresh and moist, gradually turning chestnut brown in old herbarium specimens, matt, distinctly hispid from hyaline erect hairs, 60–100 μm long, 5–10 μm thick, with indistinct septae. Upper cortex 40–60 μm thick, hyaline, composed of paraplectenchymatous tissue, with small lumina (c. 5 μm) and thin walls (c. 2 μm), becoming thicker and more sclerenchymatic near the surface. Photobiont layer 50–80 μm thick, gradually intergrading to the medulla, with 6–13 × 8–16 μm large, globose/subglobose Trebouxia cells. Medulla c. 50 μm thick, pale, composed of laxly interwoven hyphae, partly papillose. Lower cortex absent, but lower surface also hispid, with hairs up to 200 μm long. Cephalodia very common, small-foliose to small-fruticose, forming 1–5 mm large rosettes inbetween chlorobiont squamules, hispid and basically similar to the latter, but lobes smaller and 0.1–0.3 mm broad. Cyanobacteria Nostoc, 4–6 × 4–7 μm large, emerald green, sometimes grayish blue, subglobose to short-ellipsoid, often weakly angulose, arranged within 15–30 μm large glomeruli, but chain structures often distinct when liberated from their glomeruli.
Apothecia 1–3.5 mm wide, substipitate, laminal on the chlorobiont. Discs chestnut-brown on old specimens, flat to weakly convex. Thalline excipulum thin, 0.1–0.2 μm thick, weakly crenate, hispid. Epithecium light brown, c. 10 μm. Hymenium transparent, but strongly IKI+ blue, c. 70–90 μm thick. Paraphyses simple or weakly branched, densely developed and often dominating in sections, septate, weakly papillose and with swollen apices. Asci clavate, 15 × 70–90 μm, with 8 ascospores and a cap-like apical IKI + blue structure. Proper spores hyaline, non-septate, ellipsoid, 13–18 × 10–12 μm. Perispores 15–21 × 11–14 μm, verruculose when immature, later smooth, except for large apical pulvinate extensions, up to 2.5 × 4 μm, and occasional pulvinate verrucae. Hypothecium light brown, c. 70 μm thick, IKI negative.
Pycnidia scattered, marginal on chlorobiont squamules, forming brown verrucae 0.1–0.15 mm wide. Spermatia bacilliform, often weakly curved, 2–2.5 × 0.5 μm.
Chemistry: No compounds detected by TLC, and no visible melanins/pigments except in the apothecia.
Additional specimens studied (25). CHILE. XIV Región de los Ríos: Mafil, 300 m, 16 Aug. 1925, P. A. Hollermayer (BG; W 1927-384); La Unión, Llancacura, 22 Jan. 1969, M. Mahu 1626 (NY; SGO; UPS); Mahu s.n. (B 600166453; B 600157147; W 1976-09802); Valdivia, Collico, 13 Sept.1940, R. Santesson 8315 (S); Corral, Dec. 1905, R. Thaxter (B 102087; W 1935-1722); Lago Riñihue, Riñihue, 22 IX. 1940, R. Santesson 3526 (S); Curiñanco, March 7, 1948, B. Sparre 4753 (S). X Región del los Lagos: Parque Nacional Vicente Pérez Rosales. Lago Todos Los Santos. Puerto Manzano, slopes of Cordillera Derrumbe, 41° 12′ S, 72° 17′ W, 500–800 m. 11 Dec. 1986, B.J. Coppins 5013, D.J. Galloway, G. Guzmán & P.W. James (SGO; BM; CHR); Prov. Osorno, forest in valley of Río Nauto near road to Refugio Antillanca, between Laguna El Encanto and Lago Toro, 2400 ft., 14 Sept 1969, H. Imshaug 43,097 (SGO; W 1994-03668; W 1994-091844); Llanquihue Lake, 150 m, 1965, G. Follmann 16,748 (B 600192138); XI Región de Aisén: Taitao Peninsula, Península de Sisquelán, 30 m inland, 1 Feb. 1990, G. Hilsden & S. Brown 10,431 (BG); Port Grappeler (Patagonia), 1868, R.O. Cunningham (BM); Gray Harbor, 30 Nov. 1868, R.O. Cunningham (BM); Isla Berta (near Tortel), 47° 49′ 22.5″ S, 73° 47′ 50.7″, 2 Marzo 2003, J. Cuvertino 60 (SGO 149825); J. Cuvertino 58 (SGO 149729); c. 10 km E of Laguna Cofré, 46° 08.147′ S, 72° 35.600′ W, 350 m, 27 Nov. 2007, A. Elvebakk 07:038 (TROM; H); c. 5 km W of Laguna Cofré, 46° 11.259′ S, 72° 45.598′ W, 580 m, 5 Dec. 2007, A. Elvebakk 07:306 (TROM); 07:309 (TROM).
Phormopsora Elvebakk, S.G. Hong & C.H. Park, gen. nov.
MycoBank: MB 837373.
Generitype: Phormopsora isabellina (Vain.) Elvebakk, Hong & C.H. Park.
Diagnosis: Differs from Pannaria by its content of norstictic and connorstictic acids and combination of large, branched chlorobiont squamules with large, foliose cephalodia and from most species in Pannaria by a thick-walled upper cortex with small lumina, and by bullate perispores with long-apiculate apical extensions.
Etymology: From Greek “phormós” (= “mat”) and “psora” (“scale”) because of it thallus of large and often intertwining squamules.
Description: Thallus of chloromorph 2–5 cm diam., corticolous, large-squamulose, squamules up to 3 mm, irregularly branched, forming pale gray rosettes, often surrounded by a distinct prothallus. Upper cortex paraplectenchymatic, with thick walls. Cephalodia large, placodioid to foliose. Apothecia common, discs rufous brown with crenulate-striate thalline margins. Hymenium IKI+ blue, asci without internal amyloid structures. Proper spores ellipsoid, perispores gibbose, with long, filiform apical extensions.
Chemistry: norstictic and connorstictic acids.
Phormopsora isabellina (Vain.) Elvebakk, S.G. Hong & C.H. Park, comb. nov.
MycoBank: MB 837376.
≡ Psoroma isabellinum Vain. in Hedwigia 38: 188 (1899) ≡ Pannaria isabellina (Vain.) Elvebakk & Bjerke in Lichenologist 37: 48 (2005). Types: ‘Ad truncus arborum 1000 m s. m, in regionibus silvosis andini in Chili, Neger n. 94 (holotype TUR-V!, isotype M, isotype designated here) (Fig. 4).
Phormopsora isabellina was thoroughly treated (as Pannaria isabellina) by Elvebakk and Bjerke (2005), including morphology, anatomy, and chemistry, with several illustrations, including one SEM picture showing ascospores deposited on an apothecium disc. The habitat was described, and the species was shown to be common in the Lago Llanquihue and Lago Todos Los Santos areas in the X Region of Chile, as indicated by 11 collections cited from this area. In addition, two more localities were cited further north in what is now the XIV Region, whereas three additional localities were cited from the huge XI and XII Regions of Chile. For these aspects Elvebakk and Bjerke (2005) is referred to. Since then, the species has been cited from Argentina (Passo et al. 2008), from Isla Mocha in Región VIII del Bío-Bío of Chile by Quilhot et al. (2010), and from the XI Region of Aisén by Quilhot et al. (2012).
Additional specimens studied (26): CHILE. IX Región de la Araucanía: Parque Nacional Nahuelbuta, Pehuenco, 37° 36′ S, 72° 48′ W, c. 1300 m, 11 Dec. 2007, A. Elvebakk 07:326; 327 (TROM); c. 5 km W of Malalcahuello, S of the mouth of the valley Estero Huamachuco, 38° 27′ 50.5″ S, 71° 38′ 03.4″ W, 880 m, 13 Dec. 2009, A. Elvebakk 09:109 (TROM); 1.2 km S of SE boundary of Reserva Nacional Malalcahuello, 38° 34′ S, 71° 30′ W, 1400 m, 13 Dec. 2009, A. Elvebakk 07:385 (TROM); Parque Nacional Villarica, 1000 m, 4 Dec. 1978, W. Quilhot (UV); X Región de los Lagos: Ensenada am Llanquihue-See, 26 Jan 1961, F. Mattick 536 (B 28774); Petrohué Sur, mayo 1974, J. Redon 03105 (UV); Lago Yelcho, Playa Cari, 28 Nov. 1993, W. Quilhot (UV); XI Región de Aisén: NE of Puerto Aysén, 15 km S of Lago las Torres, 44° 56.721′ S, 72° 09.768′ W, 270 m, 11 Dec. 2006, A. Elvebakk 06:428A; 06:432A; 06:435; 06:432B; 06:441 (TROM); C. 13 km N of Puerto Aisén, along road 1–5 km N of laguna de los Palos, 45° 17′ S, 72° 46′ W, 40 m, 31 Jan. 1989, L. Tibell 18121a (UPS L-31042); Valle de Río Simpson, Puente Las Pizzaras, 45° 28.111′ S, 72° 18.367′ W, 9 Dec. 2006, A. Elvebakk 06:404; 06:413 (TROM); Parque Nacional Queulat, Río Ventisquero, 1 km E of Carretera Austral, 44° 28.276′ S, 72° 33.68′ W, 20 m, 11 Dec. 2006, A. Elvebakk 06:503; 06:526A (TROM); Miradór del Ventisquero, 300 m W of W end of laguna Tempanos, 44° 28.161′ S, 72° 32.673′ W, 30 m, 11 Dec. 2006, A. Elvebakk 06:506 (TROM); 19 km along the road W of Puerto Río Tranquilo (= c. 10 km W of Lago Tranquilo), 46° 37.488′ S, 72° 51.623′ W, 320 m, 28 Nov. 2007, A. Elvebakk 07:049 (TROM); 50 km N of junction Carretera Austral and road to Tortel, 2.5 km N of Puente Los Ñadis, 47° 35.208′ S, 72° 51.894′ W, 100 m, 2 Dec. 2007, A. Elvebakk 07:222 (TROM); 4 km E Caleta Tortel, along the road, and near the river, 47° 48.073′ S, 73° 29.199′ W, 10 m, 3 Dec. 2007, A. Elvebakk 07: 252 (TROM); Valle Explotadores, near Cabañas Alacalufes, c. 3 km E of Lago Bayo, 46° 30′ 26″ S, 73° 03′ 53″ W, 250 m,, 29 Nov. 2007, A. Elvebakk 07:106A (TROM); near W part of lago Bayo, 46° 29.514′ S, 73° 07.292′ W, 150 m, 29. Nov. 2007, A. Elvebakk 07:077 (TROM); XII Región de Magallanes: Comúna Cabo de Hornos, Parque Nacional Cabo de Hornos, Islas Wollaston, SE coast of Isla Grevy, Península Low, 55° 34′ 53″ S, 67° 36′ 27″, 24 Jan. 2014, W.R. Buck 62,944 (NY); Península de Brunswick, c. 13 km N of Cabo San Isidrio and c. 2.8 km S of bridge over Río San Pedro. 53° 43′ 52″ S, 70° 58′ 01″ W, 5 m, 6 Dec. 2012, A. Elvebakk 12:156 (TROM).
Discussion
When ITS and mtSSU sequences from single samples of Pannaria hispidula and P. isabellina were introduced into a combined phylogram by Passo et al. (2008), the genus Pannaria appeared as monophyletic. However, when these single sequences were integrated into new phylograms with a much broader taxon sampling, Pannaria was shown to be polyphyletic (Ekman et al. 2014; Fryday et al. 2017). In the present study, we tried to establish the phylogenetic positions of these species by including more specimens and more sequence information from the ITS, LSU, mtSSU, and MCM7 genes. Monophyly of the genera Hispidopannaria and Phormopsora was a clear conclusion from the phylogenies based on single loci or the combined dataset of ITS, LSU, mtSSU, and MCM7 (Fig. 1; Fig. S1). They formed independent phylogenetic lineages from the genus Pannaria and other genera of Pannariaceae. Considering these results, we concluded that it is reasonable to separate the genera Hispidopannaria and Phormopsora from the genus Pannaria.
Together, the two genera formed a monophyletic group by Bayesian and ML analyses of the combined dataset, but the monophyletic relationship was not maintained in phylogenies of LSU and mtSSU sequences. The relationship of the two genera with the genus Pannaria was highly variable according to the phylogenetic methods and genetic loci used in the analyses (Fig. 1; Fig. S1). In addition, the relationships among Hispidopannaria, Phormopsora, and Pannaria were changed by including or excluding specific taxa of the Pannariaceae or related taxa (data not shown), a conclusion which is valid also for other genera represented in the phylograms. Different phylogenetic markers have been used in recent phylogenetic studies on Pannariaceae, and this, as well as marker incongruence, may have led to variable and instable phylogenies of the family (Ekman and Jørgensen 2002; Passo et al. 2008; Wedin et al. 2009; Elvebakk et al. 2010, 2016; Ekman et al. 2014; Magain and Sérusiaux 2014; Lendemer et al. 2017; Fryday et al. 2017; Park et al. 2018; Marthinsen et al. 2019). The conclusion from comparing the application of single phylogenetic markers in the present study (Fig. 1; S1) is that each marker tells its own story on relationships among Pannariaceae genera. Multi-locus analyses will therefore reflect general evolutionary patterns better, and analyses involving more than the four markers used in the present study would probably improve the phylogenies. Obviously, patterns are also becoming much more stable if more than one or two samples of a studied taxon are analyzed.
In five of the six major character groups (phylogeny, gross morphology, ascus amyloidy, secondary chemistry, excipulum characters) used for circumscribing genera by Ekman et al. (2014), Hispidopannaria and Phormopsora differ from neighboring groups. In some cases, they are unique within Pannariaceae. The gross thallus morphology of Hispidopannaria is large-squamulose, with geotropically arranged squamules, also in the cyanomorph, and the squamules have erect, hispid hairs. Perispores have occasional pulvinate verrucae in combination with pulvinate apical extensions, and the asci have internal IKI+ blue apical structures, otherwise absent from Pannaria in a wide sense. Both genera have particularly well-developed cephalodia, whereas Phormopsora contains norstictic and con-norstictic acids, a unique chemistry within Pannariaceae. Its acquisition of this group of compounds is impossible to explain, as it cannot have originated from any extant relatives. Phormopsora has gibbose perispores with long, filiform, apical extensions. This type of spores is rare in Pannaria; however, it has been reported from some small-squamulose species like P. implexa and P. pholidotoides, and in foliose species such as P. athroophylla and P. patagonica (Passo and Calvelo 2011). Except for the perispore morphology of Phormopsora, these character states are rendered synapomorphic only if Hispidopannaria and Phormopsora are recognized as separate genera. Otherwise, the genus Pannaria in a wide sense will be less precisely circumscribed, with a great span in character states, and will be less clearly recognized.
The Pannaria byssoidea clade was a sister group of the genus Pannaria by the combined dataset and mtSSU, but was included in the Pannaria clade by LSU and MCM7. In contrast, it formed an independent clade by ITS2. Pannaria byssoidea differs from the remaining Pannaria species in the present phylogenetic study primarily by being squamulose, although it also possesses leprolomin without being combined with vicanicin. The species has been shown to have a close relationship with other species of Pannaria in previous studies (Passo et al. 2008; Ekman et al. 2014; Magain and Sérusiaux 2014; Fryday et al. 2017), although it is distinct from foliose Pannaria species in most of the analyses shown here. However, it is phylogenetically much more distant from Hispidopannaria and Phormopsora in the present analyses and can be left for a future study involving additional squamulose Pannaria species, without any expected interference with the present circumscriptions of Hispidopannaria and Phormopsora. No other known Pannaria s. l. species share the synapomorphies listed for Hispidopannaria and Phormopsora here.
The genus Pannaria has been cited to include as many as c. 80 species (Ekman et al. 2014). The genus also includes several subgroups, although yet not clearly circumscribed after the abandonment of a previous system of three subgenera (Ekman et al. 2014). By contrast, the two new genera are surprisingly small, although still well circumscribed. So far, we have not observed any significant variation within the new and monospecific genus Phormopsora, neither in morphology, anatomy and chemistry, nor genetically through the eight samples analyzed here. The species appears like an evolutionary dead-end, being restricted to a limited geographical area in southern South America, and only distributed within a uniform type of habitat represented by humid forests dominated by the evergreen and related species Nothofagus betuloides and N. dombeyi. Can its isolated position be explained by reproductive isolation and/or photobiont specialization?
The chlorobionts of Psoroma s.l. and tripartite Pannaria species have almost universally been identified as Myrmecia, cf. Myrmecia, or as myrmecioid, although the variation might instead be nested within Trebouxia (Park et al. 2016; Muggia et al. 2018). A restudy of the anatomy of the chlorobiont of Phormopsora isabellina now indicates that its identity is Trebouxia instead of cf. Myrmecia, as indicated by Elvebakk and Bjerke (2005). Also, both species of Hispidopannaria share what appears to be a large-celled strain of Trebouxia. The chlorobiont of Xanthopsoroma was named “cf. Myrmecia” by Elvebakk et al. (2010). A microscope-based restudy of parts of the material of Xanthopsoroma has now indicated the presence of different Trebouxia strains, both with angular (= “Myrmecia”) and papillose chloroplasts, possibly also a second genus, as well as cells of different types and sizes. However, the large-celled Trebouxia type microscoped in Hispidopannaria and Phormopsora was not found in Xanthopsoroma. In the case of cyanobionts, Elvebakk et al. (2008) showed that P. isabellina was associated with a strongly supported and exclusive clade of Nostoc, only shared by Pannaria durietzii (P. James & Henssen) Elvebakk & D.J. Galloway from New Zealand, a distantly related lichen species, although also with very large cephalodia.
In the case of P. isabellina, pycnidia have been searched for in vain, although they may have been overlooked. With mycobiont sexuality either lacking or rare, a phylogenetically exclusive Nostoc strain, and microscopically distinct chlorobionts, reproductive isolation and/or photobiont specialization may explain the particularly low evolutionary rate and lack of adaptive radiation in Phormopsora.
Like in the genus Xanthopsoroma, the unique chemistry of Phormopsora might also have had some inhibitory effects on the establishment of symbiotic life cycles in these lichens, thus contributing to maintaining isolated lineages. The phylogenetic position of Xanthopsoroma appears to be more isolated and ancient than Phormopsora and Hispidopannaria, with various basal and not yet resolved positions in several phylogenies (Elvebakk et al. 2010, 2016; Ekman et al. 2014; Magain and Sérusiaux 2014; Fryday et al. 2017), a pattern also emerging from the present multi-locus phylogeny.
Hispidopannaria hispidula also appears to be homogeneous, although a southern collection from Isla Berta, Cuvertino 60, has consistently narrower ascospores than the other specimens studied (Fig. 2c). Hispidopannaria dasyclada is a more compact species than H. hispidula. The squamules are smaller and less dissected, but thicker. These differences are so consistent, that we believe they are true, although such characters could have been modified by more sun-exposed habitats for collections of H. dasyclada, as compared with the more humid tree trunk habitats of H. hispidula. The squamules in both species are erect and imbricate, a character highlighted by Zahlbruckner (1924), when describing Psoroma dasycladum. The characteristic, erect, transparent hairs are identical in both species, but are less prominent in H. dasyclada, and present only on some lobe apices. They were not mentioned by Zahlbruckner (1924) and might have been taken for mold, which is also represented on samples available to him, or may have been omitted by mistake, as his chosen epithet means “hairy-branched.” The cephalodia were not observed by Zahlbruckner (1924) and are of the same type as those in H. hispidula. The ascospores of H. dasyclada are of the same type as those of H. hispidula, with perispores with irregularly scattered gibbae and pulvinate verrucae (Fig. 2c). However, H. hispidula has more numerous low and equally large gibbae, often with rather regular nodulose apical extensions. In H. dasyclada, the gibbae are more irregularly distributed, and in several spores, the apical extensions are very large and almost reniform, although only two of the three known collections are fertile and only parts of two apothecia were sectioned and studied. Immature perispores of both species are verrucose like in many species of Pannaria.
Suprisingly, H. dasyclada contains pannarin, whereas H. hispidula is TLC negative. Such a contrasting chemistry is unknown in tripartite groups within Pannaria, but is common within bipartite ones, see, e.g., Jørgensen and Sipman (2004). In spite of lack of molecular support, Psoroma dasycladum is recombined within the new genus Hispidopannaria here because it shares its synapomorphies of thalli composed of erect, imbricate squamules, presence of erect hyaline hairs, mature perispores with scattered gibbose and pulvinate structures instead of regularly positioned verrucae, as well as presence of apical IKI+ ascus sheet structure. Hispidopannaria dasyclada is now confirmed as one of numerous local endemics of the 3.8–4 mill. old volcanic Robinson Crusoe Island (Quinn and Woodward 2015). Based on the geological history of the island and the phylogenetic history of the Hispidopannaria clade, this taxon must have evolved by being recruited from the much older and widespread mainland population of H. hispidula. For this reason, we can hypothesize that pannarin was acquired by H. dasyclada instead of being lost by H. hispidula.
Hispidopannaria hispidula, on the other hand, does not show any indications of such a “normal” evolutionary development on the mainland during its supposedly much longer evolutionary history there. Like Phormopsora isabellina, it appears to be in a dead-end lineage. Ascospores are often produced in low quantities in H. hispidula as noted already by Hue (1906), and paraphyses often dominate much more in the hymenium than in other Pannariaceae species. Still, the species produces spermatia/conidia as a potential indication of sexual reproduction taking place within the mycobiont. Thus, there are no indications of reproductive isolation, and photobionts have not been analyzed.
These small Pannariaceae genera are examples of small and apparently old lineages which deserve closer studies exploring their evolutionary history. The only other parallel case is Joergensenia, a monospecific genus from the same geographical area (Passo et al. 2008). Although within a different major clade of Pannariaceae, it is also squamulose and strongly deviating from its foliose sister group genus, Erioderma (Ekman et al. 2014). The latter is by contrast an evolutionary success in terms of species and distribution and includes 31 species according to Jørgensen (2006) and Jørgensen et al. (2009), with evolutionary centres widely apart in the northern Andes Mountains and in Melanesia, and with a conspicuous concentration of species also in Réunion. In contrast to Hispidopannaria and Phormopsora, the two sequenced samples of Joergensenia are genetically quite different from each other, even if collected from the same geographical area (Passo et al. 2008). New molecular samples from a larger area are needed for an improved understanding of its evolutionary history.
Some monospecific genera in other families have age estimates. The widely studied family Parmeliaceae includes two old monospecific genera, Cornicularia (Schreb.) Ach. and Emodomelanelia Divakar & A. Crespo, with estimated crown ages of c. 56 Ma, and the large-disjunct genus Coelopogon Brusse & Kärnefelt, with two species, estimated age c. 45 Ma (Divakar et al. 2017). However, the sister group of Parmeliaceae is, although not in a fully resolved position, Gypsoplacaceae, a divergence with a present age estimate of 112 Ma (Divakar et al. 2017). This is a monotypic family with the monospecific genus Gypsoplaca Timdal (Timdal 1990), an amazing example of an ancient small clade.
Distribution and habitat ecology
Phormopsora isabellina is considered to be the more common of the three species dealt with here. Prior to the study by Elvebakk and Bjerke (2005), P. isabellina had only been published from two localities in Chile. This species was shown to be common in parts of the Xth Region of Chile (Los Lagos) by Elvebakk and Bjerke (2005), and with 16 new samples reported here from the the XIth Region of Aisén it can be characterized as common also there. It must still be characterized as rare or scattered in the Región XII de Magallanes, with altogether three localities known. Four localities are reported here from the Chilean Region IX of Araucanía, and its northern limit is from the Nahuelbuta National Park. Now, the species is known from 44 collections in Chile and a single one from Argentina. Phormopsora isabellina is restricted to evergreen forest, and almost all samples have been collected from trunks of the related evergreen species Nothofagus betuloides and N. dombeyi.
Hispidopannaria hispidula is restricted to temperate rainforests, from mostly moderate altitudes, and from various phorophytes, rarely specified in label information. The species has been reported from scattered localities in central southern Chile (Zahlbruckner 1933; Räsänen 1937; Galloway 1992; Quilhot et al. 2012; Rubio et al. 2013) and corresponding latitudes of Argentina (Lamb 1958). The information from Chile given by Follmann (1965) as “abundant” and “ranging from the province of Cautín to the province of Magallanes,” does not have support in his publications nor in his collections deposited at SGO, KASS, and B and studied by us.
With the localities added here, H. hispidula is known from 29 collections in Chile and three in Argentina, and should be characterized as scattered, although there is a concentration of collections from the coastal forests south of Valdivia. Except for this region (Region XIV de los Ríos) and the neighboring region to the south (Region X de los Lagos), it was previously known from two localities in the huge Region XI de Aisén further south. Now, eight collections are added from the latter region, where the species has also been collected from a deciduous Nothofagus pumilio forest (Elvebakk 07:038; 07:306; 07:308), and once (Cuvertino 60) also from bark of the conifer Pilgerodendron uviferum.
Hispidopannaria dasyclada, on the other hand, is an endemic species of Isla Robinson Crusoe of the Juan Fernández Archipelago, and was previously only known from the type locality. Two additional collections, made by G. Kunkel in 1954 and 1955, and correctly identified by H.A. Imshaug, are newly published here. This is a species growing on rock outcrops, and with its ascending squamules, it looks quite similar to squamulose species of Cladonia often growing on such habitats. The type specimens were collected on humus soil of a rock crest (“Felskamm”) from a relatively dry and sun-exposed locality at a moderate altitude. The Portezuelo collection, made c. 2 km further to the east, is from sun-exposed rocks, whereas the third one, 3–4 km further to the east, is from a shaded ravine. These localities appear to be less humid than most H. hispidula localities on the mainland.
The Portezuelo collection was made in 1954 by Kunkel near the viewpoint said to be used by Alexander Selkirk, the model of the Robinson Crusoe novel figure, more than 300 years ago. This is also the place where the picture (Fig. 3b) showing the type locality in the background was taken by the first author in 2006. The landscape here might have been much more open in 1954 due to goat grazing, and the habitats of H. dasyclada might be under threat by invasive shrubs, particularly introduced ones, see Arellano-Cataldo and Smith-Ramírez (2016). There is an obvious need to search for the species on Robinson Crusoe Island, to know its present status and hopefully obtain fresh material available for further studies.
References
Arellano-Cataldo G, Smith-Ramírez C (2016) Establishment of invasive plant species in canopy gaps on Robinson Crusoe Island. Plant Ecol 217:289–302
Culberson CF (1972) Improved conditions and new data for the identifications of lichen products by a standardized thin layer chromatography method. J Chromatogr 72:13–125
Divakar PK, Crespo A, Ekaphan K, Leavitt SD, Singh G, Schmitt I, Lumbsch HT (2017) Using a temporal phylogenetic method to harmonize family and genus-level classification in the largest clade of lichen-forming fungi. Fungal Divers 84:101–117. https://doi.org/10.1007/s13225-017-0379-z
Ekman S, Jørgensen PM (2002) Towards a molecular phylogeny for the lichen family Pannariaceae (Lecanorales, Ascomycota). Can J Bot 80:625–634
Ekman S, Wedin M, Lindblom L, Jørgensen PM (2014) Extended phylogeny and a revised generic classification of the Pannariaceae (Peltigerales, Acomycotina). Lichenologist 46:627–656
Elvebakk A, Bjerke JW (2005) Pannaria isabellina (Vain.) comb. nov., a remarkable lichen species from Chile. Lichenologist 37:47–54
Elvebakk A, Elix JA (2017) A trio of endemic New Zealand lichens: Pannaria aotearoana and P. gallowayi, new species with a new chemosyndrome, and their relationship with P. xanthomelana. Nova Hedwigia 105:167–184. https://doi.org/10.1127/nova_hedwigia/2016/0385
Elvebakk A, Papaefthimiou D, Robertsen EH, Liaimer A (2008) Phylogenetic patterns among Nostoc cyanobionts within bi- and tripartite lichens of the genus Pannaria. J Phycol 44:1049–1059
Elvebakk A, Robertsen EH, Park CH, Hong SG (2010) Psorophorus and Xanthopsoroma, two new genera for yellow-green, corticolous and squamulose lichen species, previously in Psoroma. Lichenologist 42:563–585
Elvebakk A, Hong SG, Park CH, Robertsen EH, Jørgensen PM (2016) Gibbosporina, a new genus for foliose and tripartite, Palaeotropic Pannariaceae species previously assigned to Psoroma. Lichenologist 47:3–52
Follmann G (1965) Catálogo de los líquenes de Chile. III Thelotrematales y Cyanophiliales. Rev Univ (Univ Catól Chile) 49:17–65
Fryday AM, Ertz D, Jørgensen PM (2017) Insights into the genus Austrella (Pannariaceae, Peltigerales), including a new species from the Falkland Islands. Lichenologist 49:57–65
Galloway DJ (1992) Lichens of Laguna San Rafael, Parque ‘Laguna San Rafael’, southern Chile: indicators of environmental change. Glob Ecol Biogeogr Lett 2:37–45
Grassi MM (1950) Contribución al catálogo de líquenes argentinos, I. Lilloa 24:1–290
Hue AM (1902-1901) Causerie sur les Pannaria. Bull Soc Bot France 48:xxxi–lxv
Hue AM (1906) Lichenes morphologice et anatomice disposuit (suite). Nouv Arch Mus Hist Nat Paris 8:237–272
Hue AM (1908) Lichenes morphologice et anatomice, 1. Nouv Arch Mus Hist Nat Paris 10:169–224
Jørgensen PM (2003) Conspectus familiae Pannariaceae (Ascomycetes lichenosae). Ilicifolia 4:1–79
Jørgensen PM (2006) Conspectus familiae Pannariaceae (Ascomycetes lichenosae). Revised version 2006. Ilicifolia 4:1–83 https://kipdf.com/per-m-j0-3rgensen-botanisk-institutt-universitetet-i-bergen-revised-version-cons_5ab5ebf91723dd349c81a541.html
Jørgensen PM, Andersen HL (2015) The lichen genus Psoromidium (Pannariaceae) re-evaluated, with nomenclatural notes on Degeliella and Psoromaria. Lichenologist 47:343–348
Jørgensen PM, Sipman HJM (2004) A revision of the Pannaria rubiginosa complex in South America. Nova Hedwigia 78:311–327
Jørgensen PM, van den Boom PPG, Sérusiaux E (2009) Notes on the lichen genus Erioderma in La Réunion. Cryptogam Mycol 30:263–268
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. https://doi.org/10.1093/molbev/msy096
Lamb IM (1958) La vegetación liquenica de los Parques Nacional Patagonicos. Anal Parq Nac (Buenos Aires) 7:1–188
Lanave C, Preparata G, Sacone C, Serio G (1984) A new method for calculating evolutionary substitution rates. J Mol Evol 20:86–93
de Lange PJ, Falloway DJ, Blanchon DJ, Knight A, Rolfe JR, Crowcroft GM, Hitchmough R (2012) Conservation of New Zealand lichens. New Zeal J Bot 50:303–363
de Lange PJ, Blanchon DJ, Knight A, Elix JA, Lücking R, Frogley K, Harris A, Cooper J, Rolfe J (2018) Conservation status of New Zealand indigenous lichens and lichenicolous fungi, 2018. New Zeal Threat Classif Ser 27:1–64
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Lendemer JC, Stone BH, Tripp EA (2017) Taxonomic delimitation of the rare, eastern North American endemic lichen Santessoniella crossophylla (Pannariaceae). J Torrey Bot Soc 144:459–468
Magain N, Sérusiaux E (2014) Do photobiont switch and cephalodia emancipation act as evolutionary drivers in the lichen symbiosis? A case study in the Pannariaceae. PloS ONE 9(2):e89876. https://doi.org/10.1371/journal.pone.0089876
Magain N, Sérusiaux E (2015) The lichen genus Kroswia is a synonym of Fuscopannaria (Pannariaceae). Lichenologist 47:35–42
Marthinsen G, Rui S, Timdal E (2019) OLICH: a reference library of DNA barcodes for Nordic lichens. Biod Data J 7:e36252. https://doi.org/10.3897/BDJ.7.e36252
Muggia L, Leavitt S, Barreno E (2018) The hidden diversity of lichenized Trebouxiophyceae. Phycol. 57:503–524
Nordin A (1997) Ascospore structures in Physciaceae: an ultrastructural study. Symb Bot Upsal 32(1):195–208
Nylander W (1855) Südamerikanische Flechten, gesammelt durch W. Lechler, bestimmt durch Dr. W. Nylander. Flora 43:673–675
Nylander W (1863) Synopsis methodica lichenum omnium hucusque cognitorum praemissa introductione lingua gallica tractata. Fasc. II. Martinet, Parisiis
Nylander W (1888) Lichenes fuegiae et patagoniae. Impr. Héloin & Charles, Paris 36 pp
Orange A, James PW, White FJ (2001) Microchemical methods for the identification of lichens. British Lichen Society, pp 101
Park CH, Kim EH, Noh H-J, Elvebakk A, Hong SG (2016) Diversity and biogeography of symbiotic microalgae of the lichen genus Psoroma. The 8th IAL Symposium Lichens in Deep Time, Helsinki, Finland, Aug 1–5, 2016, Abstract Book, p. 54
Park CH, Hong SG, Elvebakk A (2018) Psoroma antarcticum, a new lichen species from Antarctica and neighbouring areas. Polar Biol 41:1083–1090
Passo A, Calvelo S (2011) Pannaria byssoidea (Pannariaceae), a new squamulose species from southern South America. Bryologist 114:756–763
Passo A, Stenroos S, Calvelo S (2008) Joergensenia, a new genus to accommodate Psoroma cephalodinum (lichenized Ascomycota). Mycol Res 112:1465–1474
Quilhot W, Cuellar M, Díaz R, Riquelme F, Rubio C (2010) Estudio preliminar de la flora liquénica de Isla Mocha, sur de Chile. Gayana Bot 67:206–212
Quilhot W, Cuellar M, Díaz R, Riquelme F, Rubio C (2012) Lichens of Aisen, southern Chile. Gayana Bot 69:57–87
Quinn JA, Woodward SL (2015) Earth’s landscape: an encyclopedia of the world’s geographic features. ABC-Clio, Santa Barbara
Räsänen V (1937) Líquenes chilenos coleccionados por el R.P. Atanasio Hollermayer en 1927–1936. Rev. Univ., Univ. Catól. Chile 22:195–211
Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rubio C, Saavedra M, Cuéllar M, Díaz R, Quilhot W (2013) Epiphytic lichens of Conguillío National Park, southern Chile. Gayana Bot 70:66–81
Schmitt I, Crespo A, Divakar PK, Fankhauser JD, Herman-Sackett E, Kalb K, Nelsen MP, Nelson NA, Rivas-Plata E, Shimp AD, Widhelm T, Lumbsch HT (2009) New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia 23:35–40
Spribille T, Muggia L (2013) Expanded taxon sampling disentangles evolutionary relationships and reveals a new family in Peltigerales (Lecanoromycetidae, Ascomycota). Fungal Divers 58:171–184
Timdal E (1990) Gypsoplacaceae and Gypsoplaca, a new family and genus of squamiform lichens. Bibl Lich 38:419–427
Vainio EA (1899) Lichenes novi rarioresque. Ser II Hedwigia 38:186–190
Wedin M, Jørgensen PM, Wiklund E (2007) Massalongiaceae fam. nov., an overlooked monophyletic group among the cyanobacterial lichens (Peltigerales, Lecanoromycetes, Ascomycota). Lichenologist 39:61–67
Wedin M, Wiklund E, Jørgensen PM, Ekman S (2009) Slippery when wet: phylogeny and character evolution in the gelatinous cyanobacterial lichens (Peltigerales, Ascomycetes). Mol Phylogenet Evol 53:862–871
Weerakoon G, Aptroot A, Wedin M, Ekman S (2018) Leightoniella zeylanensis belongs to the Pannariaceae. Nord J Bot 36:e01880. https://doi.org/10.1111/njb.01880
Zahlbruckner A (1924) Die Flechten der Juan Fernandez-Inseln. Pp. 315–408 in: Skottsberg C (ed.) The Natural History of Juan Fernandez and Easter Island. Vol. II Botany 11:315–408. 2 Pl.
Zahlbruckner A (1933) Líquenes del herbario del Museo Nacional de Santiago de Chile. Rev Chil Hist Nat 37:165–170
Zoller S, Lutzoni F, Scheidegger C (1999) Genetic variation within and among populations of the threatened lichen Lobaria pulmonaria in Switzerland and implications for its conservation. Mol Ecol 8:2049–2059
Acknowledgements
The authors are indebted to the curators of the cited herbaria for loans and for the opportunity to study at their institutions, to CONAF (Corporación Nacional Forestal) for collection permits in Chile, to Eli H. Robertsen, then at the University of Tromsø, for company in the field, and Prof. Per M. Jørgensen, University of Bergen, and an anynomous referee for comments to the manuscript. This study was partly supported by Korea Polar Research Institute (Grants No. PE16020 and PE20170).
Funding
Open Access funding provided by UiT The Arctic University of Norway.
Author information
Authors and Affiliations
Contributions
Conceptualization: AE. Field and herbarium studies: AE. Phylogeny: CHP and SHG.
Writing: AE and SHG. Visualization: AE. Final manuscript discussion: AE, SHG, CHP.
Corresponding author
Additional information
Section Editor: Gerhard Rambold
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elvebakk, A., Hong, S.G. & Park, C.H. Hispidopannaria and Phormopsora, two new and small, but evolutionary old Pannariaceae lichen genera from southern South America. Mycol Progress 19, 1353–1364 (2020). https://doi.org/10.1007/s11557-020-01632-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-020-01632-1