Abstract
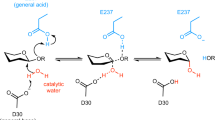
Endo-1,4-β-xylanase (EC 3.2.1.8) is a crucial enzyme that randomly cleaves the β-1,4-glycosidic linkages of the xylan backbone, releasing xylooligomers of different lengths. The three-dimensional structure of the endo-β-1,4-xylanase protein (xyl1) from Colletotrichum lindemuthianum was modeled and docked with various xylan model compounds. Docking analyses revealed significantly higher stability of C. lindemuthianum XYL1 with the xylopentaose oligomer. Residues interacting with the model oligomers at the respective enzyme active sites were found to be in accord with their role in xylan degradation. Nevertheless, docking analyses of xylanases GH11 from Colletotrichum sp. revealed significative differences in structure, integration of the substrate into the active site, and in the glutamate residues of the catalytic site involved in substrate hydrolysis; of these proteins, 36%, 60%, and 4% integrated xylotetraose, xylopentaose, and xylohexaose in the active site, respectively. Since endoxylanases GH11 from Colletotrichum species interact much more efficiently with xylopentaose and xylotetraose, and xylanases GH11 from different fungi do not seem to have the same substrate binding subsites, we propose that they are enzymes with different affinity to xylooligosaccharides. In agreement with this idea, phylogenetic analyses of xylanases from Colletotrichum sp. show four lineages, suggesting diversifying selection. Most likely, the polydiversity or structural polymolecularity of xylan in plant cell walls processed by these organisms play a determinant role in diversifying selection.








Similar content being viewed by others
References
André-Leroux G, Berrin J-G, Georis J, Arnaut F, Juge N (2008) Structure-based mutagenesis of Penicillium griseofulvum xylanase using computational design. Proteins Struct Funct Bioinform 72(4):1298–1307. doi:10.1002/prot.22029
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22(2):195–201. doi:10.1093/bioinformatics/bti770
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28(1):235–242. doi:10.1093/nar/28.1.235
Biely P (1985) Microbial xylanolytic systems. Trends Biotechnol 3(11):286–290. doi:10.1016/0167-7799(85)90004-6
Biely P, Vršanská M, Tenkanen M, Kluepfel D (1997) Endo-beta-1,4-xylanase families: differences in catalytic properties. J Biotechnol 57(1–3):151–166
Brunner PC, Torriani SF, Croll D, Stukenbrock EH, McDonald BA (2013) Coevolution and life cycle specialization of plant cell wall degrading enzymes in a hemibiotrophic pathogen. Mol Biol Evol 30(6):1337–1347. doi:10.1093/molbev/mst041
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum—current status and future directions. Stud Mycol 73(1):181–213. doi:10.3114/sim0014
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res 37(Database issue):D233–D238. doi:10.1093/nar/gkn663
Cheng YS, Chen CC, Huang CH, Ko TP, Luo W, Huang JW, Liu JR, Guo RT (2014) Structural analysis of a glycoside hydrolase family 11 xylanase from Neocallimastix patriciarum: insights into the molecular basis of a thermophilic enzyme. J Biol Chem 289(16):11020–11028. doi:10.1074/jbc.M114.550905
Choi J, Kim K-T, Jeon J, Lee Y-H (2013) Fungal plant cell wall-degrading enzyme database: a platform for comparative and evolutionary genomics in fungi and Oomycetes. BMC Genomics 14(Suppl 5):S7. doi:10.1186/1471-2164-14-s5-s7
Cole C, Barber JD, Barton GJ (2008) The Jpred 3 secondary structure prediction server. Nucleic Acids Res 36(Web Server issue):W197–W201. doi:10.1093/nar/gkn238
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29(1):3–23. doi:10.1016/j.femsre.2004.06.005
Conejo-Saucedo U, Cano-Camacho H, López-Romero E, Villa-Rivera MG, Lara-Márquez A, Zavala-Páramo MG (2016) Cloning and characterization of an endo-β-1,4-xylanase gene from Colletotrichum lindemuthianum and phylogenetic analysis of similar genes from phytopathogenic fungus. Afr J Microbiol Res 10(32):1292–1305. doi:10.5897/ajmr2016.8185
Cooper RM, Longman D, Campbell A, Henry M, Lees PE (1988) Enzymic adaptation of cereal pathogens to the monocotyledonous primary wall. Physiol Mol Plant Pathol 32:33–47
Crooks GE, Hon G, Chandonia J-M, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14(6):1188–1190. doi:10.1101/gr.849004
Davies G, Henrissat B (1995) Structures and mechanisms of glycosyl hydrolases. Structure 3(9):853–859
de Lemos Esteves F, Ruelle V, Lamotte-Brasseur J, Quinting B, Frère J-M (2004) Acidophilic adaptation of family 11 endo-β-1,4-xylanases: modeling and mutational analysis. Protein Sci : Publ Protein Soc 13(5):1209–1218. doi:10.1110/ps.03556104
Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD (2012) The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13(4):414–430. doi:10.1111/j.1364-3703.2011.00783.x
Degefu Y, Lohtander K, Paulin L (2004) Expression patterns and phylogenetic analysis of two xylanase genes (htxyl1 and htxyl2) from Helminthosporium turcicum, the cause of northern leaf blight of maize. Biochimie 86(2):83–90. doi:10.1016/j.biochi.2004.01.001
Ellouze OE, Loukil S, Marzouki MN (2011) Cloning and molecular characterization of a new fungal xylanase gene from Sclerotinia sclerotiorum S2. BMB Rep 44(10):653–658. doi:10.5483/BMBRep.2011.44.10.653
Gan P, Ikeda K, Irieda H, Narusaka M, O’Connell RJ, Narusaka Y, Takano Y, Kubo Y, Shirasu K (2013) Comparative genomic and transcriptomic analyses reveal the hemibiotrophic stage shift of Colletotrichum fungi. New Phytol 197(4):1236–1249. doi:10.1111/nph.12085
Geer LY, Marchler-Bauer A, Geer RC, Han L, He J, He S, Liu C, Shi W, Bryant SH (2010) The NCBI BioSystems database. Nucleic Acids Res 38(Database issue):D492–D496. doi:10.1093/nar/gkp858
Gilkes NR, Henrissat B, Kilburn DG, Miller RC Jr, Warren RA (1991) Domains in microbial beta-1, 4-glycanases: sequence conservation, function, and enzyme families. Microbiol Rev 55(2):303–315
Gille C, Fähling M, Weyand B, Wieland T, Gille A (2014) Alignment-Annotator web server: rendering and annotating sequence alignments. Nucleic Acids Res 42(Web Server issue):W3–W6. doi:10.1093/nar/gku400
Gómez S, Payne AM, Savko M, Fox GC, Shepard WE, Fernandez FJ, Cristina Vega M (2016) Structural and functional characterization of a highly stable endo-β-1,4-xylanase from Fusarium oxysporum and its development as an efficient immobilized biocatalyst. Biotechnol Biofuels 9(1):191. doi:10.1186/s13068-016-0605-z
Gruber K, Klintschar G, Hayn M, Schlacher A, Steiner W, Kratky C (1998) Thermophilic xylanase from Thermomyces lanuginosus: high-resolution X-ray structure and modeling studies. Biochemistry 37(39):13475–13485. doi:10.1021/bi980864l
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723. doi:10.1002/elps.1150181505
Hakulinen N, Turunen O, Jänis J, Leisola M, Rouvinen J (2003) Three-dimensional structures of thermophilic β-1,4-xylanases from Chaetomium thermophilum and Nonomuraea flexuosa. Eur J Biochem 270:1399–1412. doi:10.1046/j.1432-1033.2003.03496.x
Herron SR, Benen JA, Scavetta RD, Visser J, Jurnak F (2000) Structure and function of pectic enzymes: virulence factors of plant pathogens. Proc Natl Acad Sci U S A 97(16):8762–8769
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755
Hyde KD, Cai L, McKenzie EHC, Yang YL, Zhang JZ, Prihastuti H (2009) Colletotrichum: a catalogue of confusion. Fungal Divers 39:1–17
Jänis J, Pulkkinen P, Rouvinen J, Vainiotalo P (2007) Determination of steady-state kinetic parameters for a xylanase-catalyzed hydrolysis of neutral underivatized xylooligosaccharides by mass spectrometry. Anal Biochem 365(2):165–173. doi:10.1016/j.ab.2007.03.034
Jommuengbout P, Pinitglang S, Kyu KL, Ratanakhanokchai K (2009) Substrate-binding site of family 11 xylanase from Bacillus firmus K-1 by molecular docking. Biosci Biotechnol Biochem 73(4):833–839
King BC, Waxman KD, Nenni NV, Walker LP, Bergstrom GC, Gibson DM (2011) Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol Biofuels 4:4. doi:10.1186/1754-6834-4-4
Korb O, Stützle T, Exner TE (2009) Empirical scoring functions for advanced protein−ligand docking with PLANTS. J Chem Inf Model 49(1):84–96. doi:10.1021/ci800298z
Krengel U, Dijkstra BW (1996) Three-dimensional structure of endo-1,4-beta-xylanase I from Aspergillus niger: molecular basis for its low pH optimum. J Mol Biol 263(1):70–78. doi:10.1006/jmbi.1996.0556
Krissinel E, Henrick K (2004) Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr Sect D Biol Crystallogr 60(Pt 12 Pt 1):2256–2268. doi:10.1107/s0907444904026460
Krissinel E, Henrick K (2005) Multiple alignment of protein structures in three dimensions. In: Berthold RM, Glen RC, Diederichs K, Kohlbacher O, Fischer I (eds) Computational life sciences, vol 3695. Lecture notes in computer science. Springer, Berlin, pp 67–78. doi:10.1007/11560500_7
Kulkarni N, Lakshmikumaran M, Rao M (1999a) Xylanase II from an alkaliphilic thermophilic Bacillus with a distinctly different structure from other xylanases: evolutionary relationship to alkaliphilic xylanases. Biochem Biophys Res Commun 263(3):640–645. doi:10.1006/bbrc.1999.1420
Kulkarni N, Shendye A, Rao M (1999b) Molecular and biotechnological aspects of xylanases. FEMS Microbiol Rev 23(4):411–456
Kumar L, Dutt D, Tapas S, Kumar P (2013) Purification, bio-chemical characterization, homology modeling and active site binding mode interactions of thermo-alkali-tolerant β-1,4 endoxylanase from Coprinus cinereus LK-D-NCIM-1369. Biocatalysis Agric Biotechnol 2(3):267–277. doi:10.1016/j.bcab.2013.04.004
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947–2948. doi:10.1093/bioinformatics/btm404
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26(2):283–291. doi:10.1107/S0021889892009944
Leggio LL, Jenkins J, Harris GW, Pickersgill RW (2000) X-ray crystallographic study of xylopentaose binding to Pseudomonas fluorescens xylanase A. Proteins 41(3):362–373
Martínez-Anaya C, Balcázar-López E, Dantán-González E, Folch-Mallol JL (2008) Celulasas fúngicas: Aspectos biológicos y aplicaciones en la industria energética. Rev Latinoam Microbiol 50:119–131
Melo F, Feytmans E (1998) Assessing protein structures with a non-local atomic interaction energy. J Mol Biol 277(5):1141–1152. doi:10.1006/jmbi.1998.1665
O’Connell RJ, Bailey JA (1988) Differences in the extent of fungal development, host cell necrosis and symptom expression during race-cultivar interactions between Phaseolus vulgaris and Colletotrichum lindemuthianum. Plant Pathol 37(3):351–362. doi:10.1111/j.1365-3059.1988.tb02085.x
Paës G, Tran V, Takahashi M, Boukari I, O’Donohue MJ (2007) New insights into the role of the thumb-like loop in GH-11 xylanases. Protein Eng Design Select 20(1):15–23. doi:10.1093/protein/gzl049
Paës G, Berrin JG, Beaugrand J (2012a) GH11 xylanases: structure/function/properties relationships and applications. Biotechnol Adv 30(3):564–592. doi:10.1016/j.biotechadv.2011.10.003
Paës G, Cortés J, Siméon T, O’Donohue MJ, Tran V (2012b) Thumb-loops up for catalysis: a structure/function investigation of a functional loop movement in a GH11 xylanase. Comput Struct Biotechnol J 1:e201207001. doi:10.5936/csbj.201207001
Polizeli ML, Rizzatti AC, Monti R, Terenzi HF, Jorge JA, Amorim DS (2005) Xylanases from fungi: properties and industrial applications. Appl Microbiol Biotechnol 67(5):577–591. doi:10.1007/s00253-005-1904-7
Sabini E, Wilson KS, Danielsen S, Schuelein M, Davies GJ (2001) Oligosaccharide binding to family 11 xylanases: both covalent intermediate and mutant product complexes display (2,5)B conformations at the active centre. Acta Crystallogr Sect D Biol Crystallogr 57(Pt 9):1344–1347
Sapag A, Wouters J, Lambert C, de Ioannes P, Eyzaguirre J, Depiereux E (2002) The endoxylanases from family 11: computer analysis of protein sequences reveals important structural and phylogenetic relationships. J Biotechnol 95(2):109–131
Schneider TD, Stephens RM (1990) Sequence logos: a new way to display consensus sequences. Nucleic Acids Res 18(20):6097–6100
Sunna A, Antranikian G (1997) Xylanolytic enzymes from fungi and bacteria. Crit Rev Biotechnol 17(1):39–67. doi:10.3109/07388559709146606
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30(12):2725–2729. doi:10.1093/molbev/mst197
Thomsen R, Christensen MH (2006) MolDock: a new technique for high-accuracy molecular docking. J Med Chem 49(11):3315–3321. doi:10.1021/jm051197e
Törrönen A, Rouvinen J (1995) Structural comparison of two major endo-1,4-xylanases from Trichoderma reesei. Biochemistry 34(3):847–856
Törrönen A, Harkki A, Rouvinen J (1994) Three-dimensional structure of endo-1,4-beta-xylanase II from Trichoderma reesei: two conformational states in the active site. EMBO J 13(11):2493–2501
van Gunsteren WF, Billeter SR, Eising AA, Hünenberger PH, Krüger PK, Mark AE, Scott WRP, Tironi IG (1996) Biomolecular simulation: the {GROMOS96} manual and user guide. Hochschuleverlag AG an der ETH Zürich, Zürich, Switzerland
Vandermarliere E, Bourgois TM, Rombouts S, Van Campenhout S, Volckaert G, Strelkov SV, Delcour JA, Rabijns A, Courtin CM (2008) Crystallographic analysis shows substrate binding at the −3 to +1 active-site subsites and at the surface of glycoside hydrolase family 11 endo-1,4-beta-xylanases. Biochem J 410(1):71–79. doi:10.1042/bj20071128
Vardakou M, Dumon C, Murray JW, Christakopoulos P, Weiner DP, Juge N, Lewis RJ, Gilbert HJ, Flint JE (2008) Understanding the structural basis for substrate and inhibitor recognition in eukaryotic GH11 xylanases. J Mol Biol 375(5):1293–1305. doi:10.1016/j.jmb.2007.11.007
Wan Q, Zhang Q, Hamilton-Brehm S, Weiss K, Mustyakimov M, Coates L, Langan P, Graham D, Kovalevsky A (2014) X-ray crystallographic studies of family 11 xylanase Michaelis and product complexes: implications for the catalytic mechanism. Acta Crystallogr Sect D Biol Crystallogr 70(Pt 1):11–23. doi:10.1107/s1399004713023626
Wong KK, Tan LU, Saddler JN (1988) Multiplicity of beta-1,4-xylanase in microorganisms: functions and applications. Microbiol Rev 52(3):305–317
Zhao Z, Liu H, Wang C, Xu JR (2013) Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genomics 14:274. doi:10.1186/1471-2164-14-274
Acknowledgements
The authors thank the financial support provided by Secretaría de Educación Pública-Consejo Nacional de Ciencia y Tecnología (SEP-CONACyT), México (project 2012-01-182755 to María G. Zavala-Páramo), Coordinación de la Investigación, Universidad Michoacana de San Nicolás de Hidalgo (project 2014-2015 to Horacio Cano-Camacho), Consejo Nacional de Ciencia y Tecnología, México, for scholarship number 209148 granted to Ulises Conejo-Saucedo and scholarship number 233565 granted to Maria G. Villa-Rivera, and Universidad Nacional Autónoma de México for postdoctoral fellowship program 2012-14 granted to Alicia Lara-Márquez.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Marc Stadler
Rights and permissions
About this article
Cite this article
Conejo-Saucedo, U., Cano-Camacho, H., Villa-Rivera, M.G. et al. Protein homology modeling, docking, and phylogenetic analyses of an endo-1,4-β-xylanase GH11 of Colletotrichum lindemuthianum . Mycol Progress 16, 577–591 (2017). https://doi.org/10.1007/s11557-017-1291-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11557-017-1291-3