Abstract
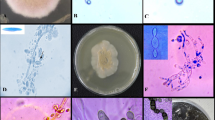
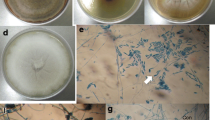
In a survey of coprophilous fungi in Italy, two interesting ascomycetes were recovered from hedgehog dung. These fungi showed a sexual morph characterized by gymnothecia, globose reticulate ascospores, a Malbranchea asexual state, and a keratinolytic ability, and were identified morphologically and molecularly as Auxarthron umbrinum and A. concentricum of the Onygenaceae. The history, ecology, and morphology of the genus Auxarthron as a whole, and of A. umbrinum and A. concentricum in particular, are revised. A preliminary evaluation of the antagonistic properties of A. umbrinum and A. concentricum against phytopathogenic fungi has been performed in dual cultures. Growth inhibition of some plant pathogenic fungi was recorded, and the effects were growth medium dependent. When solid-state fermentation (SSF) substrate of both Auxarthron isolates has been submitted to extraction, both the organic extract residues (n-hexane and CH2Cl2) and the lyophilized aqueous phases were used for the antibiotic test against plant pathogenic fungi. Significant antifungal activity was obtained by organic fractions and aqueous residue of A. concentricum against Alternaria brassicicola and Botrytis cinerea, whereas A. umbrinum appeared to be less effective. The analysis of ITS nrDNA sequences suggests that an extensive phylogenetic revision of the genus Auxarthron is necessary.










Similar content being viewed by others
References
Ajello L, Varsavsky E, Sotgiu G, Mazzoni A, Mantovani A (1965) Survey of soils for human pathogenic fungi from the Emilia-Romagna region of Italy. I. Isolation of keratinophilic fungi. Mycopathologia 26:65–71
Al-Musallam AA (1989) Distribution of keratinophilic fungi in desert soil of Kuwait. Mycoses 32:296–302. doi:10.1111/j.1439-0507.1989.tb02247.x
Alvi KA, Rabenstein J (2004) Auxarthrol A and auxarthrol B: two new tetrahydoanthraquinones from Auxarthron umbrinum. J Ind Microbiol Biotechnol 31:11–15. doi:10.1007/s10295-003-0106-5
Apinis AE (1964) Revision of British Gymnoascaceae. Mycol Pap 96:1–56
Baroncelli R (2012) Colletotrichum acutatum sensu lato: from diversity study to genome analysis. PhD thesis, University of Warwick, Coventry, UK
Bills GF, Gloer JB, An Z (2013) Coprophilous fungi: antibiotic discovery and functions in an underexplored arena of microbial defensive mutualism. Curr Opin Microbiol 16:549–565. doi:10.1016/j.mib.2013.08.001
Błyskal B (2009) Fungi utilizing keratinous substrates. Int Biodeterior Biodegrad 63:631–653. doi:10.1016/j.ibiod.2009.02.006
Bowman BH, White TJ, Taylor JW (1996) Human pathogeneic fungi and their close nonpathogenic relatives. Mol Phylogenet Evol 6:89–96. doi:10.1006/mpev.1996.0061
Cano J, Guarro J, Figueras MJ (1987) Some keratinophilic fungi from Spain. Mycopathologia 100:163–167. doi:10.1007/BF00437043
Caretta G, Piontelli E (1975) Isolation of keratinophilic fungi from soil in Pavia, Italy. Sabouraudia 13:33–37. doi:10.1080/00362177585190061
Clark BR, Murphy CD (2009) Biosynthesis of pyrrolylpolyenes in Auxarthron umbrinum. Org Biomol Chem 7:111–116. doi:10.1039/b813236d
Clark BR, Capon RJ, Lacey E, Tennant S, Gill JH (2006) Polyenylpyrroles and polyenylfurans from an Australian isolate of the soil ascomycete Gymnoascus reessii. Org Lett 8:701–704. doi:10.1021/ol052880y
Clark BR, O’Connor S, Fox D, Leroy J, Murphy CD (2011) Production of anticancer polyenes through precursor-directed biosynthesis. Org Biomol Chem 9:6306–6311. doi:10.1039/c1ob05667k
Crous PW, Wingfield MJ, Guarro J, Cheewangkoon R, van der Bank M, Swart WJ, Stchigel AM, Cano-Lira JF, Roux J, Madrid H, Damm U, Wood AR, Shuttleworth LA, Hodges CS, Munster M, de Jesús Yáñez-Morales M, Zúñiga-Estrada L, Cruywagen EM, de Hoog GS, Silvera C, Najafzadeh J, Davison EM, Davison PJ, Barrett MD, Barrett RL, Manamgoda DS, Minnis AM, Kleczewski NM, Flory SL, Castlebury LA, Clay K, Hyde KD, Maússe-Sitoe SN, Chen S, Lechat C, Hairaud M, Lesage-Meessen L, Pawłowska J, Wilk M, Sliwińska-Wyrzychowska A, Mętrak M, Wrzosek M, Pavlic-Zupanc D, Maleme HM, Slippers B, Mac Cormack WP, Archuby DI, Grünwald NJ, Tellería MT, Dueñas M, Martín MP, Marincowitz S, de Beer ZW, Perez CA, Gené J, Marin-Felix Y, Groenewald JZ (2013) Fungal Planet description sheets: 154–213. Persoonia 31:188–296. doi:10.3767/003158513X675925
Currah RS (1985) Taxonomy of the Onygenales: Arthrodermaceae, Gymnoascaceae, Myxotrichaceae and Onygenaceae. Mycotaxon 24:1–216
Deshmukh SK, Verekar SA, Shrivastav A (2010) The occurrence of keratinophilic fungi in selected soils of Ladakh (India). Nat Sci 2:1247–1252. doi:10.4236/ns.2010.211151
Dewan MM, Ghisalbertib EL, Rowland C, Sivasithamparam K (1994) Reduction of symptoms of take-all of wheat and rye-grass seedlings by the soil-borne fungus Sordaria fimicola. Appl Soil Ecol 1:45–51. doi:10.1016/0929-1393(94)90022-1
Doveri F (2004) Fungi Fimicoli Italici. A.M.B.-Fondazione Centro Studi Micologici, Vicenza
Doveri F, Pecchia S, Vergara M, Sarrocco S, Vannacci G (2012) A comparative study of Neogymnomyces virgineus, a new keratinolytic species from dung, and its relationships with the Onygenales. Fungal Divers 52:13–34. doi:10.1007/s13225-011-0120-2
Doveri F, Sarrocco S, Vannacci G (2013) Studies on three rare coprophilous plectomycetes from Italy. Mycotaxon 124:279–300. doi:10.5248/124.279
Emmons CW (1954) Isolation of Myxotrichum and Gymnoascus from the lungs of animals. Mycologia 46:334–338
Fang Z, Liao PC, Yang YL, Yang FL, Chen YL, Lam Y, Hua KF, Wu SH (2010) Synthesis and biological evaluation of polyenylpyrrole derivatives as anticancer agents acting through caspases-dependent apoptosis. J Med Chem 53:7967–7978. doi:10.1021/jm100619x
Filipello Marchisio V (1986) Keratinolytic and keratinophilic fungi of children’s sandpits in the city of Turin. Mycopathologia 94:163–172. doi:10.1007/BF00454595
Filipello Marchisio V (2000) Keratinophilic fungi: their role in nature and degradation of keratinic substrates. In: Kushwaha RKS, Guarro J (eds) Biology of dermatophytes and other keratinophilic fungi. Revista Iberoamericana de Micología, Bilbao, pp 86–92
Filipello Marchisio V, Fusconi A, Rigo S (1994) Keratinolysis and its morphological expression in hair digestion by airborne fungi. Mycopathologia 127:103–115. doi:10.1007/BF01103066
Gloer GB (2007) Applications of fungal ecology in the search for new bioactive natural products. In: Kubicek CP, Druzhinina IS (eds) Environmental and microbial relationships. The mycota IV, 3rd edn. Springer-Verlag, Berlin Heidelberg, pp 257–283
Gloer JB, Truckenbrod SM (1988) Interference competition among coprophilous fungi: production of (+)-isoepoxydon by Poronia punctata. Appl Envron Microbiol 54:861–864
Griffin DM (1960) Fungal colonization of sterile hair in contact with soil. Trans Br Mycol Soc 43:583–596. doi:10.1016/S0007-1536(60)80048-4
Guarro J, Punsola L, Calvo MA (1981) Keratinophilic fungi from soil of Tarragona, Catalunya. Mycopathologia 76:69–71. doi:10.1007/BF00443752
Guarro J, Gené J, Stchigel AM, Figueras MJ (2012) Atlas of soil Ascomycetes. CBS Biodiversity Series 10, Utrecht
Hosoe T, Fukushima K, Takizawa K, Miyaji M, Kawai KI (1999) Three pyrrolyloctatetraenyl-α-pyrones from Auxarthron conjugatum. Phytochemistry 52:459–463. doi:10.1016/S0031-9422(99)00237-X
Howard DH (2003) Pathogenic fungi in humans and animals, 2nd edn. Marcel Dekker, New York
Hubka V, Dobiasova S, Lyskova P, Mallatova N, Chlebkova J, Skorepova M, Kubatova A, Dobias R, Chudickova M, Kolarik M (2013) Auxarthron ostraviense sp. nov., and A. umbrinum associated with non-dermatophytic onychomycosis. Med Mycol 51:614–624. doi:10.3109/13693786.2013.770608
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI Publishing, Wallingford
Kuehn HH (1955a) Observations on Gymnoascaceae. I. Myxotrichum uncinatum and a new species of Myxotrichum. Mycologia 47:533–545. doi:10.2307/3755667
Kuehn HH (1955b) Observations on Gymnoascaceae. II. Two new species of Myxotrichum. Mycologia 47:878–890. doi:10.2307/3755509
Kuehn HH (1959) A preliminary survey of the Gymnoascaceae. II. Mycologia 51:665–692. doi:10.2307/3755896
Kushwaha RKS (2000) The genus Chrysosporium, its physiology and biotechnological potential. In: Kushwaha RKS, Guarro J (eds) Biology of dermatophytes and other keratinophilic fungi. Revista Iberoamericana de Micología, Bilbao, pp 66–76
Matarese F, Sarrocco S, Gruber S, Seidl-Seiboth V, Vannacci G (2012) Biocontrol of Fusarium head blight: interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 158:98–106. doi:10.1099/mic.0.052639-0
McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, Cowley AP, Lopez R (2013) Analysis Tool Web services from the EMBL-EBI. Nucleic Acids Res 41:W597–W600. doi:10.1093/nar/gkt376
Nannizzi A (1926) Ricerche sui rapporti morfologici e biologici tra Gymnoascaceae e dermatomiceti. Ann Mycol 24:85–129
Onuma J (2007) Diversity of coprophilous fungi, antagonism against plant pathogenic fungi, and secondary metabolites of Ascodesmis macrospora and Sordaria fimicola. PhD thesis, Kasetsart University, Thailand
Orr GF (1977) Another genus of the Gymnoascaceae with swollen septa on peridial elements. Mycotaxon 5:283–290
Orr GF, Kuehn HH (1972) Notes on Gymnoascaceae. II. Some Gymnoascaceae and keratinophilic fungi from Utah. Mycologia 64:55–72. doi:10.2307/3758013
Orr GF, Kuehn HH, Plunkett OA (1963) A new genus of the Gymnoascaceae with swollen peridial septa. Can J Bot 41:1439–1456. doi:10.1139/b63-126
Richardson MJ (2001) Diversity and occurrence of coprophilous fungi. Mycol Res 105:387–402. doi:10.1017/S0953756201003884
Richardson MJ, Watling R (1997) Keys to fungi on dung. British Mycological Society, Stourbridge
Ronquist F, Huelsenbeck JP (2003) MrBates 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi:10.1093/bioinformatics/btg180
Samson RA (1972) Notes on Pseudogymnoascus, Gymnoascus and related genera. Acta Bot Neerl 21:517–527
Sarrocco S, Diquattro S, Avolio F, Cimmino A, Puntoni G, Doveri F, Evidente A, Vannacci G (2015) Bioactive metabolites from new or rare fimicolous fungi with antifungal activity against plant pathogenic fungi. Eur J Plant Pathol 142:61–71. doi:10.1007/s10658-014-0589-0
Scott JA, Untereiner WA (2004) Determination of keratin degradation by fungi using keratin azure. Med Mycol 42:239–246. doi:10.1080/13693780310001644680
Sharma R, Rajak RC, Pandey AK (2006) Keratinophilic Ascomycetes from central India. J Mycopathol Res 44:27–38
Sharma M, Sharma R, Rao VM (2011) In vitro biodegradation of keratin by dermatophytes and some soil keratinophiles. Afr J Biochem Res 5:1–6
Sigler L, Carmichael JW (1976) Taxonomy of Malbranchea and some other Hyphomycetes with arthroconidia. Mycotaxon 4:349–488
Sigler L, Hambleton S, Flis AL, Paré JA (2002) Auxarthron teleomorphs for Malbranchea filamentosa and Malbranchea albolutea and relationships within Auxarthron. Stud Mycol 47:111–122
Skinner SJ, Tsuneda A, Currah RS (2006) Morphology and development of the reticuloperidial ascomata of Auxarthron conjugatum. Mycologia 98:447–454. doi:10.3852/mycologia.98.3.447
Smith AL, Ramsbottom J (1916) New or rare microfungi. Trans Br Mycol Soc 5:422–433. doi:10.1016/S0007-1536(14)80044-2
Solé M, Cano J, Guarro J (2002a) Molecular phylogeny of Amauroascus, Auxarthron, and morphologically similar onygenalean fungi. Mycol Res 106:388–396. doi:10.1017/S0953756202005750
Solé M, Cano J, Stchigel AM, Guarro J (2002b) Two new species of Auxarthron morphologically and genetically close to A. kuehnii. Stud Mycol 47:103–110
Sugiyama M, Mikawa T (2001) Phylogenetic analysis of the non-pathogenic genus Spiromastix (Onygenaceae) and related onygenalean taxa based on large subunit ribosomal DNA sequences. Mycoscience 42:413–421. doi:10.1007/BF02464337
Sugiyama M, Summerbell RC, Mikawa T (2002) Molecular phylogeny of onygenalean fungi based on small subunit (SSU) and large subunit (LSU) ribosomal DNA sequences. Stud Mycol 47:5–23
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 30:2725–2729. doi:10.1093/molbev/mst197
Toro MA, Ferrari B, Pino J, Piontelli E (2007) Onygenales (Eurotiomycetes, Ascomycota) queratinofilicos en suelos de establecimientos educacionales urbanos y rurales de la V región, Chile. Bol Micol 22:1–8
Udagawa S (1966) Notes on some Japanese Ascomycetes III. Trans Mycol Soc Japan 7:91–98
Ulfig K, Guarro J, Cano J, Gené J, Vidal P, Figueras MJ, Łukasik W (1998) A preliminary study of the occurrence of actidione-resistant fungi in sediments of Catalonian river mouths (Spain). I. Keratinolytic fungi and related Onygenales. Mycopathologia 141:143–151. doi:10.1023/A:1006978032246
Untereiner WA, Scott JA, Naveau FA, Currah RS, Bachewich J (2002) Phylogeny of Ajellomyces, Polytolypa and Spiromastix (Onygenaceae) inferred from rDNA sequence and non-molecular data. Stud Mycol 47:25–35
Untereiner WA, Scott JA, Naveau FA, Sigler L, Bachewich J, Angus A (2004) The Ajellomycetaceae, a new family of vertebrate-associated Onygenales. Mycologia 96:812–821. doi:10.2307/3762114
von Arx JA (1971) On Arachniotus and related genera of the Gymnoascaceae. Persoonia 6:372–380
von Arx JA (1974) The genera of fungi sporulating in pure culture. Cramer, Vaduz
von Arx JA (1977) Notes on Gymnoascaceae. Persoonia 9:393–400
von Arx JA (1987) A re-evaluation of the Eurotiales. Persoonia 13:273–300
Watanabe T (1991) Evaluation of Sordaria spp. as biocontrol agents against soilborne plant diseases caused by Pythium aphanidermatum and Dematophora necatrix. Jpn J Phytopathol 57:680–685. doi:10.3186/jjphytopath.57.680
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, New York, pp 315–322. doi:10.1016/B978-0-12-372180-8.50042-1
Yamagishi Y, Matsuoka M, Odagawa A, Kato S, Shindo K, Mochizuki J (1993) Rumbrin, a new cytoprotective substance produced by Auxarthron umbrinum. I. Taxonomy, production, isolation and biological activities. J Antibiot (Tokyo) 46:884–887. doi:10.7164/antibiotics.46.884
Acknowledgments
This paper is dedicated to Maurizio Forti, who recently passed away. The authors would like to thank Riccardo Antonelli for his support with the scanning electron microscope and Lucia Levorato for providing part of the material used in this study.
Conflict of interest
All authors state that they have no conflicts of interest with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Franz Oberwinkler
Rights and permissions
About this article
Cite this article
Sarrocco, S., Diquattro, S., Baroncelli, R. et al. A polyphasic contribution to the knowledge of Auxarthron (Onygenaceae). Mycol Progress 14, 112 (2015). https://doi.org/10.1007/s11557-015-1128-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11557-015-1128-x