Abstract
Background
Reduction of peritendinous adhesions after injury and repair has been the subject of extensive prior investigation. The application of a circumferential barrier at the repair site may limit the quantity of peritendinous adhesions while preserving the tendon’s innate ability to heal. The authors compare the effectiveness of a type I/III collagen membrane and a collagen-glycosaminoglycan (GAG) resorbable matrix in reducing tendon adhesions in an experimental chicken model of a “zone II” tendon laceration and repair.
Methods
In Leghorn chickens, flexor tendons were sharply divided using a scalpel and underwent repair in a standard fashion (54 total repairs). The sites were treated with a type I/III collagen membrane, collagen-GAG resorbable matrix, or saline in a randomized fashion. After 3 weeks, qualitative and semiquantitative histological analysis was performed to evaluate the “extent of peritendinous adhesions” and “nature of tendon healing.” The data was evaluated with chi-square analysis and unpaired Student’s t test.
Results
For both collagen materials, there was a statistically significant improvement in the degree of both extent of peritendinous adhesions and nature of tendon healing relative to the control group. There was no significant difference seen between the two materials. There was one tendon rupture observed in each treatment group. Surgical handling characteristics were subjectively favored for type I/III collagen membrane over the collagen-GAG resorbable matrix.
Conclusion
The ideal method of reducing clinically significant tendon adhesions after injury remains elusive. Both materials in this study demonstrate promise in reducing tendon adhesions after flexor tendon repair without impeding tendon healing in this model.
Similar content being viewed by others
Introduction
Intrasynovial flexor tendon injuries remain an ongoing treatment challenge in hand surgery. Flexor tendon surgery has advanced greatly since excision and secondary grafting was the standard of care [3]. Modern techniques, beginning with the sentinel work of Kleinert et al., have been fine-tuned with regard to suture technique, handling of the tendon sheath/pulleys, multiple local and systemic adjuvant modalities, and postoperative rehabilitation protocols [11, 20].
Tendon healing and the formation of adhesions to the surrounding tissue with the resultant poor functional excursion have been the topic of much study. Tendons have been described to heal by both intrinsic and extrinsic processes. Intrinsic healing involves the function of tenocyte repopulation and adherence. The extrinsic pathway involves fibroblasts from the surrounding tissue aiding in the formation of scar tissue at the site of injury. These processes are not mutually exclusive for the ultimate result in tendon healing [15].
Studies have demonstrated that up to 20 % of patients following tendon repair will have limiting or debilitating tendon adhesions despite adequate technique and appropriately timed therapy [15]. Up to 10 % of these will require secondary procedures for this complication [21]. Peritendinous adhesions were once thought to be an integral part of tendon healing, but with a more thorough understanding of flexor tendon biology, we now know this to be untrue [4, 15].
Current management of clinically appreciable adhesions includes secondary operations and continued aggressive therapy. Many adjunctive modalities have been explored as a means of primarily reducing adhesions. Attempts at systemic treatment have included NSAIDS [12, 17], steroids, and 5-fluorouracil [1, 16], all aimed at reducing inflammation and altering the cellular and molecular milieu in which the tendon heals. These are not without adverse systemic effects. Local application of “adhesion barriers” with hyaluronic acid, colloid gel, collagen, metal tubes, cellophane, various polymeric hydrocarbons, silicone sheeting, and many other “off-the-shelf” products has been attempted in animal and human models with variable success [2, 6, 7, 9, 13, 18, 21, 24].
Autologous Chondrocyte Implantation-Maix™ collagen membrane (Matricel, Herzogenrath, Germany) is a resorbable, biocompatible product of purified porcine-derived collagen type I/III membrane used clinically in Europe as a delivery vehicle for chondrocytes to repair articular cartilage. TenoGlide® Tendon Protector Sheet (Integra LifeSciences Corporation, Plainsboro, NJ) is a permeable matrix of type I collagen and glycosaminoglycan (GAG), which is resorbable and biocompatible. These products were selected for evaluation in their ability to reduce tendon adhesions in the primary repair of an iatrogenic tendon injury. They are compared histologically to a control group in a previously validated chicken tendon model [13]. We postulate that these products will reduce the histologically identifiable tendon adhesions without impeding tendon healing.
Materials and Methods
Experimental Design and Animal Model
A total of 18 female adult Leghorn chickens (Gallus domesticus) weighing 1.0 to 2.0 kg, and 17–19 months of age at the time of the operations, were used. The study was approved and monitored by the Institutional Animal Care and Use Committee at our institution, ensuring that all institutional and national guidelines for the care and use of laboratory animals were followed.
The animals were numerically identified and randomized to either right or left leg surgery. Each leg was then treated with the three techniques. The second, third, and fourth toes were randomly assigned to one of the three treatment groups. The groups were type I/III collagen membrane, collagen-GAG resorbable matrix, and an untreated surgical control group. Randomization was facilitated with a Microsoft Excel spreadsheet (Microsoft, Redmond, WA).
The surgeon remained blinded to the assignment of the toe until the tendon had been repaired. Due to the nature of the treatments, the surgeon was unable to be blinded to treatment group.
Preparation of the Type I/III Collagen Membrane and Collagen-GAG Resorbable Matrix
In anticipation of surgical implantation, the type I/III collagen membrane was handled using sterile technique at all times. The product has a polarity, with a “smooth side” and a “rough side.” The smooth side was marked with surgical marking pen for later identification. The product was hydrated in standard saline irrigation solution for a period of at least 2 min prior to surgical implantation. During the surgical application, the product was rehydrated using the saline solution as needed. The “smooth side” was applied toward the tendon substance.
Collagen-GAG resorbable matrix was handled using aseptic surgical technique at all times and used according to manufacturer’s instructions. The product was removed from outer packaging, and the polyethylene sheets encasing the implant material were carefully removed due to product fragility. The product was placed in a standard saline irrigation solution for 1–2 min to remove the storage buffer solution prior to implantation.
Surgical Procedure
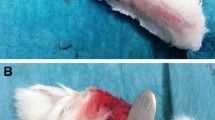
Each female adult Leghorn chicken was anesthetized using isoflurane via nosecone and digital block was performed with 1 % lidocaine to the second, third, and fourth toes. The surgical sites were cleaned with chlorhexidine solution and prepped with Betadine solution for surgery. Aseptic technique was maintained throughout the procedure. Under tourniquet control and loupe magnification, a midlateral incision was made on the second, third, and fourth toes distal to the chiasm of the superficial tendon. The tendon sheath was then excised in a circumferential fashion for a length of 1 cm to expose 5 mm of tendon proximal and distal to the repair site. The flexor profundus tendon to each of the toes was sharply divided using a scalpel within the flexor sheath window (Fig. 1). It was then repaired using a 5-0 polypropylene monofilament suture with a modified Kessler technique.
The tendon was then treated, according to the randomization spreadsheet generated, with type I/III collagen membrane, collagen-GAG resorbable matrix, or no treatment.
The animals treated with type I/III collagen membrane (group 1) and collagen-GAG resorbable matrix (group 2) had the previously prepared material applied to the tendon repair site in a circumferential manner so that the test material encompassed the repair site. The material was then sutured to itself using a 6-0 polypropylene suture on a tapered needle in a running fashion (Figs. 2 and 3 demonstrate application of products). The animals included in the surgical control group received no further intervention.
All surgical sites were closed with interrupted 4-0 PDS sutures. The wounds were then padded and immobilized with a below-knee cast. Animals were allowed to ambulate freely in these casts.
Histological Evaluation
At 3 weeks (21 days), animals were euthanized and casting material was removed. All toes were separated from the leg and marked according to the randomization schedule. The specimens were placed in labeled containers with 10 % neutral buffered formalin solution and stored for 24 h prior to sectioning and staining.
Slide preparation and standard hematoxylin/eosin staining was performed at our designated laboratory. The longitudinally sectioned slides were then evaluated by a board-certified veterinary pathologist who performed qualitative and semiquantitative analysis of the specimen using light microscopy. The pathologist performing the analysis was blinded to the group assignments.
Each tendon was examined for the nature of tendon healing and the degree of adhesions to the surrounding soft tissue. Longitudinal sections through the tendon were taken from each specimen submitted and evaluated based on a modification of a previously established grading system [13] described in Tables 1 and 2.
Statistical Analysis
A total of 51 toes were used in the analysis, with each group consisting of 17 sites. Chi-square analysis comparing no, minimal, and mild adhesions to moderate and marked adhesions between the treatment groups and the untreated control group was performed. Additionally, unpaired Student’s t tests were performed comparing type I/III collagen membrane and collagen-GAG resorbable matrix to the control group. A p value of less than 0.05 was considered statistically significant.
Our initial groups consisted of 18 surgical sites. With regard to extent of peritendinous adhesions, one site was excluded from analysis in each group. One group 1 site was excluded due to an infection. Two sites, one each from groups 2 and 3, were excluded due to tendon rupture. With regard to nature of tendon healing, three tendons in group 1 and six tendons in group 2 were unable to be evaluated due to an inability of the veterinary pathologist to definitively identify the site of the tendon injury on histologic evaluation. These were not excluded from peritendinous adhesion analysis.
Results
The analysis demonstrated statistically significant improvements in the degree of peritendinous adhesions and nature of tendon healing relative to untreated controls for both the type I/III collagen membrane (p < 0.01) and collagen-GAG resorbable matrix (p < 0.01) groups (Fig. 4).
The type I/III collagen membrane group demonstrated a mild or lesser degree of adhesions (score of 2 or less) as defined by a 4-point evaluation scale in 94 % (p < 0.01) of tendons analyzed [13]. In the collagen-GAG resorbable matrix group, 100 % of sites were characterized as having mild or lesser adhesions (score of 2 or less; p < 0.01). In the control tendons, 0 % of sites were described as mild or lesser adhesions (score of 2 or less; p < 0.01) (see Figs. 5, 6, and 7).
Type I/III collagen membrane present between the epitenon and surrounding tissue without significant adhesion. The approximate width of tendon is noted by thin black bars. The thick arrow demonstrates product surrounding tendon. Thin brackets demonstrate a thin layer of scar tissue around the product but not to the tendon repair site. Empty holes represent suture sites
Collagen-GAG resorbable matrix present surrounding the epitenon without significant adhesion to surrounding tissue. The approximate width of tendon is noted by thin black bars. The thick arrow points to the tendon product with no appreciable scar tissue in contact with tendon surface. Empty holes represent suture sites
With regard to the nature of tendon healing, the type I/III collagen membrane demonstrated 50 % and collagen-GAG resorbable matrix demonstrated 64 % scores of good or excellent relative to 0 % in the untreated control group (Fig. 8). Only 14 of 17 specimens in the type I/III collagen membrane group and 11 of 17 specimens in the collagen-GAG resorbable matrix group could be evaluated for nature of tendon healing due to inability to conclusively identify the repair site despite repeat histologic sections. All 17 control specimens were evaluated histologically.
There were no statistically significant differences between the type I/III collagen membrane and collagen-GAG resorbable matrix groups in regard to either peritendinous adhesion or tendon healing.
The type I/III collagen membrane scaffold and the collagen-GAG resorbable matrix material were present and located appropriately between the epitenon and surrounding tissue in all histological samples at the 3-week mark.
Of note, there was a mild-to-marked mononuclear inflammatory response to the type I/III collagen membrane relative to the collagen-GAG resorbable matrix and control groups. The clinical significance of this is uncertain at this time. Type I/III collagen membrane had more favorable surgical handling characteristics from the perspective of the operating surgeon/principal investigator.
Discussion
The complex interplay of tissues involved in healing of an intrasynovial flexor tendon injury is becoming better understood. Injury to the tendon often involves damage to multiple tissue types including the skin, subcutaneous tissue, digital fascial structures, synovial sheath, and the tendon itself, each with unique healing properties [22]. When a tendon is lacerated in “no man’s land,” the normal intrinsic blood supply and nutrition to the tendon is disrupted in that portion of the tendon [14]. This is further complicated by introduction of an inflammatory milieu with metabolic activity that has the capacity to impede extrinsic nutrition to the tendon.
These cellular and subcellular interactions are occurring in a patient with multiple variables impacting their ability to heal the injury. These may include the severity of soft tissue injury and resultant pain, smoking, diabetes, poor compliance with mobility protocols, variable suture technique, and surgeon skill. If a surgeon has the ability to modify healing at the focal point of this complex injury, superior results may be attained.
The purpose of a tendon barrier is to allow extrinsic nutrition to the tendon while modulating the deposition of adhesive collagen fibers between the epitenon and surrounding damaged synovium or subcutaneous tissues. This will allow tendon glide and limit collagenous bulk within the repair site. The ideal properties of an adhesion barrier include the ability to remain at the site of injury during the early healing process, resorbability, biocompatibility, and favorable handling characteristics to enable easy application [5].
The collagen membranes evaluated in this study are thought to have properties that allow for the diffusion of growth factors and cytokines necessary for the intrinsic healing process [23]. The type I/III collagen membrane and collagen-GAG resorbable matrix have demonstrated the aforementioned characteristics, though not all were specifically evaluated in this study. We were able to demonstrate that both barriers significantly reduced adhesion of the surrounding soft tissue to the epitenon without impeding healing at the site of the repair.
In contrast to the early mobility protocols commonly used in the clinical setting, experimental animals were subjected to immobilization for 3 weeks prior to sacrifice and analysis. The intent was to create conditions favorable to adhesion formation. The materials tested may yield better results when combined with an appropriate program of controlled mobilization.
Currently, few studies utilize the chicken model and compare barrier products. Siddiqi et al. [19] demonstrated that the application of hydroxyapatite or alumina sheaths after excision of the native flexor sheath and tendon repair was protective of tendon adhesions using both mobility scores and histologic evaluation at 3, 6, and 12 weeks. Isik et al. [8] and Kakarum et al. [10] used hyaluronic acid/carboxymethylcellulose sheaths and demonstrated favorable adhesion barrier characteristics for both tendon repair and tenolysis at various time intervals. In most animal models, the fewer histologic adhesions tend to correlate with biomechanical improvement in motion. Our model utilized a barrier of collagen products to demonstrate comparable results at a similar interval at least with regard to histologic analysis.
With regard to product handling, the collagen-GAG resorbable matrix was found to be bulkier and displayed more difficulty holding sutures, whereas the type I/III collagen membrane is thinner and is more easily sutured. The type I/III collagen membrane was favored over collagen-GAG resorbable matrix by the operating surgeon in terms of handling characteristics.
While the grading system of adhesions and tendon healing are not objective tests, there was a clear distinction between treated groups in our study and the control group. In addition, the veterinary pathologist was blinded throughout the study process. The authors acknowledge the limitations of this study including the lack of biomechanical testing. The purpose of this study was, however, to analyze from a histological perspective the adhesion reduction efficacy of these products. Biomechanical testing will be employed in a future investigation.
Both type I/III collagen membrane and collagen-GAG resorbable matrix demonstrate a statistically significant reduction in the degree of peritendinous adhesions while not impeding tendon healing compared to untreated tendon repairs. Collagen-based barriers may be a useful adjunct to standard repair and rehabilitation protocols to reduce postoperative tendon adhesions and improve clinical outcomes. Further study is needed to identify the ideal tendon adhesion barrier.
References
Akali A, Khan U, Khaw PT, McGrouther AD. Decrease in adhesion formation by a single application of 5-fluorouracil after flexor tendon injury. Plast Reconstr Surg. 1999;103:151–8.
Akasaka T, Nishida J, Araki S, Shimamura T, Amadio PC, An KN. Hyaluronic acid diminishes the resistance to excursion after flexor tendon repair: an in vitro biomechanical study. J Biomech. 2005;38:503–7.
Bunnell S. Repair of tendons in the fingers and description of two new instruments. Surg Gynecol Obstet. 1918;26:103–10.
Caulfield RH, Maleki-Tabrizi A, Patel H, Coldham F, Mee S, Nanchahal J. Comparison of zones 1 to 4 flexor tendon repairs using and unabsorbable four strand core sutures. J Hand Surg Eur. 2008;33:412–7.
Ferguson RE, Rinker B. The use of a hydrogel sealant on flexor tendon repairs to prevent adhesion formation. Ann Plast Surg. 2006;56(1):54–8.
Hagberg L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: a controlled clinical trial. J Hand Surg [Am]. 1992;17A:132–6.
Hanff G, Abrahamsson SO. Matrix synthesis and cell proliferation in repaired flexor tendons within E-PTFE reconstructed flexor tendon sheaths. J Hand Surg. 1996;21:642–6.
Isik S, Ozturk S, Gurses S, Yetmez M, Guler MM, Selmanpakoglu N, et al. Prevention of restrictive adhesions in primary tendon repair by HA-membrane: experimental research in chickens. Br J Plast Surg. 1999;52(5):373–9.
Jones ME, Burnett S, Southgate A, Sibbons P, Grobbelaar AO, Green CJ. The role of human-derived fibrin sealant in the reduction of postoperative flexor tendon adhesion formation in rabbits. J Hand Surg (Br). 2002;27B:78–82.
Karakarum G, Byukbebeci O, Kalender M, Gulec A. Seprafilm interpositions for preventing adhesion formation after tenolysis. An experimental study on the chicken flexor tendons. J Surg Res. 2003;113(2):195–200.
Kleinert HE, Kutz JE, Ashbell TS, Martinez E. Primary repair of lacerated flexor tendons in “no man’s land”. J Bone Joint Surg. 1967;49A:577.
Kulick MI, Brazlow R, Smith S, Hentz VR. Injectable ibuprofen: preliminary evaluation of its ability to decrease pretendinous adhesions. Ann Plast Surg. 1984;13:459–67.
Liu Y, Skardal A, et al. Prevention of peritendinous adhesions using a hyaluronan-derived hydrogel film following partial-thickness flexor tendon injury. J Orthop Res. 2008;26(4):562–9.
Lundborg G, Rank F. Experimental intrinsic healing of flexor tendons based upon synovial fluid nutrition. J Hand Surg [Am]. 1978;3:21.
Manske PR. Flexor tendon healing (review). J Hand Surg (Br). 1988;13:237–45.
Moran SL, Ryan CK, Orlando GS, Pratt CE, Michalko KB. Effects of 5-fluorouracil on flexor tendon repair. J Hand Surg [Am]. 2000;25A:242–51.
Nishimura K, Shimanuki T, deZerega GS. Ibuprofen in the prevention of experimentally induced postoperative adhesions. Am J Med. 1984;77:102–6.
Porat S, Rousso M, Shoshan S. Improvement of gliding function of flexor tendons by topically applied enriched collagen solution. J Bone Joint Surg (Br). 1980;62B:208–13.
Siddiqi NA, Hamada Y, Ide T, Akamatsu N. Effects of hydroxyapatite and alumina sheaths on postoperative peritendinous adhesions in chickens. J Appl Biomater. 1995;6(1):43–53.
Small JO, Brennen MD, Colville J. Early active motion following flexor tendon repair in zone II. J Hand Surg (Br). 1989;14(4):383–91.
Tang JB. Clinical outcomes associated with flexor tendon repair. Hand Clin. 2005;21:199–210.
Wong J, Lui Y, Kapacee Z, Kadler K, Ferguson M, McGrouther D. The cellular biology of flexor tendon adhesion formation: an old problem in a new paradigm. Am J Pathol. 2009;175(5):1938–51.
Yannas IV. Studies on the biological activity of the dermal regeneration template. Wound Repair Regen. 1998;6:518.
Zhao C, Sun YL, Amadio PC, Tanaka T, Ettema AM, An KN. Surface treatment of flexor tendon autografts with carbodiimide-derivatized hyaluronic acid. An in vivo canine model. J Bone Joint Surg Am. 2006;88A:2181–91.
Acknowledgments
This study was funded by a grant from Genzyme, A Sanofi Company.
Conflict of Interest
Authors John B. Turner and Brian D. Rinker disclose that the funding for the study was from a grant by Genzyme. Authors Rubina L. Corazzini and Timothy J. Butler disclose that they currently maintain employment with Genzyme.
Author David S. Garlick discloses that his laboratory was compensated for tissue histopathology of the study.
Statement of Human and Animal Rights
All procedures and animal care was approved by our Institutional Animal Care and Use Committee. All national and institutional guidelines for the care and use of laboratory animals were followed.
Statement of Informed Consent
Informed consent was not required.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Turner, J.B., Corazzini, R.L., Butler, T.J. et al. Evaluating adhesion reduction efficacy of type I/III collagen membrane and collagen-GAG resorbable matrix in primary flexor tendon repair in a chicken model. HAND 10, 482–488 (2015). https://doi.org/10.1007/s11552-014-9715-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11552-014-9715-x