Abstract
Purpose
Virtual reality (VR) allows for an immersive and interactive analysis of imaging data such as computed tomography (CT) and magnetic resonance imaging (MRI). The aim of this study is to assess the comprehensibility of VR anatomy and its value in assessing resectability of pancreatic ductal adenocarcinoma (PDAC).
Methods
This study assesses exposure to VR anatomy and evaluates the potential role of VR in assessing resectability of PDAC. Firstly, volumetric abdominal CT and MRI data were displayed in an immersive VR environment. Volunteering physicians were asked to identify anatomical landmarks in VR. In the second stage, experienced clinicians were asked to identify vascular involvement in a total of 12 CT and MRI scans displaying PDAC (2 resectable, 2 borderline resectable, and 2 locally advanced tumours per modality). Results were compared to 2D standard PACS viewing.
Results
In VR visualisation of CT and MRI, the abdominal anatomical landmarks were recognised by all participants except the pancreas (30/34) in VR CT and the splenic (31/34) and common hepatic artery (18/34) in VR MRI, respectively. In VR CT, resectable, borderline resectable, and locally advanced PDAC were correctly identified in 22/24, 20/24 and 19/24 scans, respectively. Whereas, in VR MRI, resectable, borderline resectable, and locally advanced PDAC were correctly identified in 19/24, 19/24 and 21/24 scans, respectively. Interobserver agreement as measured by Fleiss κ was 0.7 for CT and 0.4 for MRI, respectively (p < 0.001). Scans were significantly assessed more accurately in VR CT than standard 2D PACS CT, with a median of 5.5 (IQR 4.75–6) and a median of 3 (IQR 2–3) correctly assessed out of 6 scans (p < 0.001).
Conclusion
VR enhanced visualisation of abdominal CT and MRI scan data provides intuitive handling and understanding of anatomy and might allow for more accurate staging of PDAC and could thus become a valuable adjunct in PDAC resectability assessment in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
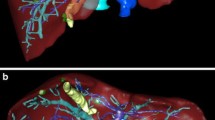
Virtual reality (VR) allows the viewer to experience a computer-generated environment interactively [1, 2]. Recently, a picture archiving and communication system (PACS) compatible VR software has been developed (Specto VR™), capable of rendering, segmenting, and displaying cross-sectional medical imaging such as Computed Tomography (CT) or Magnetic Resonance Imaging (MRI) in real-time [3]. In essence, this software can be used to import a cross-sectional imaging dataset directly from PACS and render this image data into a freely interactive 3D model, allowing detailed study of the data in a VR environment. The original dataset can be superimposed simultaneously with the rendered volume model by the usage of a cutting plane. (Fig. 1) This software has previously been validated in various settings, including ophthalmological imaging as well as Magnetic Resonance Cholangio-Pancreatography (MRCP) [4, 5]. In these studies, the VR application was shown to be safe, was well tolerated, improved the anatomical understanding, and even potentially introduced a clinical benefit when used as a preoperative tool for trainee surgeons.
Visualisation of the basic display options a, b Images from different modalities can be loaded into the VR environment. The models can then be placed, moved and rotated as desired. c Sectioning of the 3D model is possible using the shown cutting plane d The cutting plane can be switched, so that the matching section of the respective modality is shown
Even though VR visualisation is currently explored extensively, translation into daily clinical practice on a wide scale has not yet occurred [6, 7]. In the future, it is likely that VR enhanced visualisation of abdominal CT and MRI data will gain further importance [8]. In the past, the usage of VR anatomical models to support learning and understanding of anatomy has been shown to be beneficial [9]. However, recognising anatomical structures in VR CT and MRI imaging of real patients is a necessary cornerstone to facilitate the clinical implementation of this technology. In the future, application of VR technology to assess complex medical images such as vascular involvement of pancreatic ductal adenocarcinoma (PDAC) could improve surgical and oncological treatment planning. In PDAC, the surgical resectability decision is mainly based on two-dimensional cross-sectional image assessment, which is a challenge in clinical practice due to notoriously low inter-observer agreement (7.2%-30% for CT scans, Fleiss κ range 0.282–0.555) [10, 11]. According to the current National Comprehensive Cancer Network (NCCN) guidelines, three categories of resectability exist: Resectable (R), borderline resectable (BR) and locally advanced (LA) disease [12]. (Table 1).
Of note, the resectability of PDAC likely represents a clinical continuum rather than a clear cut-off, further adding to the complexity or resectability assessment. Nonetheless, current guidelines recommend contrast-enhanced CT or MR imaging to assess local resectability, and VR software might add potential benefit by allowing the viewer to freely interact with 3D rendered cross-sectional imaging as well as viewing the original dataset.
In this study, we aim firstly to assess the general capability of clinicians from various backgrounds, both in terms of clinical experience as well specialisation, to understand the displayed anatomy with immersive, 3D-rendered VR CT and MRI visualisation. In the second step, this technology is tested for its usability as a tool to assess vascular involvement and ultimately, the resectability of PDAC by expert abdominal surgeons and radiologists.
Methods
This prospective study was conducted according to the Declaration of Helsinki in Basel, Switzerland at Clarunis (University Centre for Gastrointestinal and Liver Disease), consisting of the abdominal surgery units of St. Claraspital (SCS) and the University Hospital in Basel as well as the Royal Free Hospital, London, UK. The Ethics Committee of Northwestern and Central Switzerland approved the use of patient data (Ethikkommission Nordwest und Zentralschweiz, EKNZ 2021-00457; AO_2021-00053). The study participants who were medical professionals provided written informed consent. Patients contributing data either signed a written informed consent form issued by the EKNZ or data was used if they had previously signed the institutions general research consent.
Participants
In the first study step, all clinicians from the named institution were eligible to participate. In the second study step, abdominal surgeons and radiologists who had completed their training or were undergoing specialist Hepatobiliary and Pancreatic surgical subspecialisation were eligible for study participation. Study participants were recruited via a written invitation to the surgical, medical, radiological, and gastroenterological departments or via personal invitation. Study participation was voluntary without financial compensation. Study procedures were explained verbally, and participants gave written and oral informed consent prior to study inclusion. Each participant was assigned a participation number. The literature on usability testing determined the number of study participants [13].
Study procedures
The study was divided into two steps. In the first study step, a pre-test survey to obtain demographical data, professional experience and previous exposure to VR was completed by the participants. Each study participant was individually exposed to the VR experiments. After fitting of a head-mounted display (HMD) to the participants’ head, they were allowed to acclimatise to the VR environment. Detailed instructions of the usage of the system were given. Then, a 3D VR CT model was displayed. To measure correct detection of requested anatomical structures, participants were asked to highlight specific anatomical structures with a cursor built into the VR system that could be observed on the computer screen, and the correctness of the answer was recorded. The same procedure was repeated for the 3D VR MRI model. The study personnel monitored all actions of the participants in the VR surroundings on an additional screen. A short post-test survey was handed out upon completion of the VR anatomy identification task with two open questions were asked: (1) What did you like most? (2) What did you like least?. No limitation to the length of the answers was given.
In the second study step, imaging data from 34 patients with a pathology or cytology proven PDAC and MRI or CT staging were selected from the internal hospital cancer database, with a comparable number of R, BR, and LA cases on imaging as defined by the NCCN [12].
Clinical reports, preoperative imaging, postoperative outcomes as well as multidisciplinary team (MDT) outcomes were collected for all patients included in the study. MDT discussions were chaired by the clinical leads or their deputies of the surgical, oncological, radiological, and radiooncological departments, who had to reach consensus regarding resectability and treatment of the patients. Mean work-experience post-completion of clinical training of the senior members of each units was 22 years. Abdominal surgeons and radiologists were recruited for demonstration of a set of 6 individually randomly selected VR CT and 6 VR MRI scans. Each participant assessed 2 scans with R, BR and LA PDAC per imaging modality. The participants were allowed free usage of all the VR software’s features, including the cutting plane and no time limit was given. Time measured from beginning of image display to final verdict of vascular involvement and resectability status was recorded by the study personnel. Two months after the conduction of these experiments, study participants were shown the same CT scans in standard 2D PACS, serving as control group. Correct assessment of vascular involvement (R, BR, LA) as well as time to answer were measured. The candidates’ answers were compared to the official imaging reports from the respective radiology departments as well as the MDT decision reports.
VR software and equipment and radiological imaging
The VR application used (Specto VR™, Version 4.0, Specto Medical, Basel, Switzerland) allows imaging data to be imported and displayed in real-time, enhanced by real-time ray casting. Specto VR™ displays volume rendered images at 90 frames per second per eye. Colour transfer functions can be freely be adjusted to visualise different tissues. A cutting plane with free adjustability was provided to display the original dataset (cross-sectional slices) on demand, and to interact with the original dataset. Free rotation in every direction of the VR model as well as zooming in or out is possible. (Fig. 1) To run this application, an ASUS ROG Zephyrus GX501GI-EI005T (15.60″; full HD; Intel Core i7-8750H, 16 GB; 512 GB hard-drive; and graphics processing unit Nvidia GTX1080 MaxQ) laptop computer was used. Two VR HMD were used (HTC Vive, Xindian District, New Taipei City, Taiwan) and HP Mixed Reality (Hewlett-Packard, Palo Alto, California, USA).
In the used cross-sectional imaging, individual voxel size was: X: 0.799 (mean) ± 0.178, Y: 0.799 (mean) ± 0.178, Z (slice thickness): 2.433 ± 0.751. The Interslice gap was −0.461 (mean) ± 0.560. In case of overlapping slice reconstruction, negative interslice gap values are indicated.
Used MRI models were rendered T1 images with fat suppression using radio-frequency–spoiled 3D GRE sequences. For CT, the used sequences were late arterial or portal venous and as arterial, depending on the protocol used in the respective radiological department. Study participants were allowed to freely study the arterial as well as portal venous or late arterial phases, depending on availability. Appendix 1.
Statistical analysis
All variables are expressed as the median and interquartile range, or counts (percentages), unless otherwise specified. The descriptive nature of the data was confirmed by an independent statistician. Microsoft Excel™ v16.68 (Microsoft, Redmond, Washington, USA) was used for descriptive analysis. Correlation analysis was performed using the Spearman’s rank correlation for paired and independent variables. Inter-rater agreement analysis was conducted with Fleiss Kappa. Differences among proportions derived from categorical data were compared using the Pearson Chi Square (χ2) test. Two-group comparison of normally distributed data was performed by the Student’s t test. Comparison between small sample sizes and non-normal distributed data was performed with the Mann–Whitney U test. Statistical analysis was performed with R version 3.3.2 (The R Project for Statistical Computing, GNU General Public License version 2) and R Studio version 1.0.44 (RStudio) with the graphical user interface, rBiostatistics.com [14].
Results
Subjects
In the first study step, the majority of subjects were abdominal surgeons (31/34), 3 were radiologists, specialised in abdominal imaging (3/34). Median age was 37 years (IQR 37–45) and fourteen were female (14/34). Median work experience was 11 years (IQR 0.33–17). The majority had finished their training (24/34). A minority had previous experience with VR (9/34), the rest of the participants was VR-naïve (25/34).
In the second study step, 9 subjects were abdominal surgeons and 3 were radiologists. One was female, and median age was 40 years (IQR 36–46). Median work experience was 14 years (IQR 9–20) and all participants had completed their training. In the 2D PACS control group, a total of 5 surgeons participated with a median age of 39 (IQR 34–41) and a median work experience of 13 years (IQR 6–15). Control group and study group did not differ significantly in age (p = 0.927) and work experience (p = 0.783).
Recognisability of abdominal anatomy in VR CT and VR MRI
In VR CT, all 34 participants were able to detect all the tested solid organs (liver, spleen, kidneys) with a lower rate for the pancreas (30/34). All vascular structures were correctly identified by all of the participants (aorta, inferior vena cava, portal vein, coeliac trunk, portal vein, splenic artery, and common hepatic as well as the superior mesenteric artery).
In VR MRI, all the participants were able to detect all of the queried solid organs. All the participants correctly identified the vascular structures listed apart from the common hepatic artery (18/34). (Table 2).
Vascular involvement of PDAC and assessment of resectability in VR
All twelve participants reached a verdict for each scan regarding vascular tumour contact, except in one case. A lack of response was recorded as an erroneous answer. A correct response was defined as consistency with the written MDT assessment. Median time for the assessment of VR CT and MRI scans was 185 s (IQR 138–212) and 116 s (IQR 95–152), respectively. Median assessment time for 2D PACS CT was 110 s (IQR 60–154). Median correct answers per participant (maximum 6 per imaging modality) in VR CT and MRI were 5.5 (IQR 4.75–6) and 5 (IQR 4.75–5), respectively. In 2D PACS CT viewing, median correct answers per participants were 3 (IQR 2–3). Compared to the 3D VR group, significantly fewer correct answers were given in the 2D PACS CT group whilst interacting a significantly shorter amount of time (p < 0.001).
In VR CT, R, BR, and LA PDAC was identified in 22/24 (92%), 20/24 (83%) and 19/24 (79%) scans, respectively. In VR MRI, R, BR, and LA PDAC was identified in 19/24 (79%), 19/24 (79%) and 21/24 (88%) scans, respectively. In 2D PACS CT, R, BR and LA PDAC was identified in 6/10 (60%), 2/10 (20%) and 4/10 (40%) scans, respectively. Five out of six participants preferred VR CT over VR MRI for the assessment of resectability. Correlation analysis between work experience and number of correct answers did not reveal a correlation with ρ = −0.18 (p = 0.41). Interobserver agreement as measured with Fleiss κ was 0.7 (p < 0.001) for VR CT, indicating substantial agreement. For VR MRI, κ was 0.4 (p < 0.001), indicating fair agreement. For 2D PACS CT, κ was 0.04 (p = 0.48), indicating slight agreement.
The details of the differing interpretations of scans are discussed in Table 3 (Fig. 2, 3).
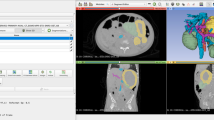
CT model of locally advanced pancreatic cancer. a Section through the CT model of a patient with LA pancreatic cancer. b The cutting plane can be placed in any desired angle. c Overview of an axial section: (1) Aorta (2) Celiac trunk (3) Common hepatic artery (4) Splenic artery. d Light–Dark contrast can be adapted for each model. The tumour is clearly visible on this section (5). e, f Tumour encasement of vessels is seen in various sections from different angles: (6) Aorta (7) Celiac trunk (8) Superior mesenteric artery
MR model of resectable pancreatic cancer. Similar to the CT model, the same assessment can be made for an MR model. a Section through the MR model of a patient with resectable pancreatic cancer. b Example of an axial section without visible tumour mass: (1) Aorta (2) Biliary duct (3) Superior mesenteric artery
Participant feedback
Participant feedback in the form of a post-survey was collected in free text form. 31 out of 34 participants provided an answer to the question “what did you like the most?” and 20 out of 34 participants provided an answer to the question “what did you like the least?”. Mainly, participants enjoyed the ease of handling (10/34), quality of 3D imaging (10/34), improved understanding of anatomy (7/34) and the free movability of the reconstructed model (5/34). In contrast, the image resolution (9/34) and weight of the headset (8/34) were the main criticisms issued by the participants. (Table 4).
In the second study step, the majority of the participants stated that the anatomy displayed was easy to understand (11/12), that VR can improve patient treatment (12/12), can help to anticipate intraoperative difficulties (9/12), and represented overall an enjoyable experience (12/12). No significant correlation between positive answers and performance as measured by correct answers was found for above named items with ρ = 0.05 (p = 0.88), ρ = 0.12 (p = 0.69), ρ = −0.21 (p = 0.52), and ρ = −0.12 (p = 0.7), respectively.
Discussion
In this pilot study, 3D VR-enhanced abdominal CT and MRI were able to display the anatomy in an understandable way, both for junior and experienced medical professionals. The VR experience was received positively by the tested study population. Experienced surgeons and radiologists were able to assess the vascular involvement and, ultimately, the resectability of PDAC in the majority of cases presented. Direct comparison between 3D VR enhanced and standard 2D PACS viewing of CT imaging displaying PDAC showed significantly higher accuracy in the VR group.
The first part of the study aimed at assessing the understandability of 3D VR enhanced cross-sectional abdominal imaging. There are only a few studies that directly assess the comprehensibility of VR-enhanced abdominal CT or MRI anatomy [15, 16]. Previously, a VR-enhanced CT scan was reported in a feasibility study, however, the software automated segmentation and PACS import was not available. For the implementation of VR as a clinical adjunct, the accuracy and comprehensibility of VR-enhanced visualisation will need to be further scrutinised to guarantee patient safety. This study aims to lay a cornerstone for this purpose, as most participants, including junior doctors could reliably identify the displayed anatomy. In the past, this VR software has been evaluated as a tool to teach anatomy, indicating that anatomical learning was perceived as more efficient and engaging when compared to the standard anatomy learning with models or books [17]. A recently published meta-analysis including a total of 15 randomised trials that evaluated the efficacy of VR-supported anatomy teaching, concluded that post-VR intervention anatomical test scores were significantly higher when compared with other teaching methods [9]. In these studies, anatomical VR models were used, but not PACS imported imaging datasets. Overall, these results align with previously published results from our group, showing that VR MRCP led to faster and more accurate anatomical understanding when compared to printed MRCP scans [5].
Although the participant’s feedback was largely positive, and more participants provided positive than negative feedback, a few criticisms must be addressed. The main criticism of the VR experience was a perceived lack of image resolution. The used VR software imports isovoxels from the original dataset in a 1:1 fashion, thus the resolution is limited not by the VR software, but by the original dataset. In VR, the model can be zoomed in and magnified to an extent where the VR model becomes larger than the scanned person in real life. By comparison, most screens used are relatively small, leading to the viewers perception of sufficient image resolution as compared to the magnification possible in the VR system. In the future, artificial intelligence might be introduced to improve appearances and resolution of imaging, however, this may come with new difficulties and problems as the original dataset could be altered [18]. Another comment issued by the participants was the weight of the headset. Although a recognised problem, recent advances in hardware development will likely render this issue obsolete and should not be seen as a deterrent for future use of VR in a clinical setting [19].
In the second part of the study, this VR software was evaluated as a tool to assess the presence or absence and extent of vascular contact, and ultimately the resectability in PDAC. In general, inter-observer agreement is known to be low in PDAC imaging and has been reported to be as low as 7.2–30% [10, 11]. This is also true for experienced radiologists, who show only slightly improved interobserver agreement compared to their less experienced counterparts [11]. Given these numbers, our reported results in the 3D VR group show a relatively high interobserver agreement. This is also reflected in the Fleiss κ range of 0.4–0.7 in our cohort, compared to the κ range of 0.282–0.555 reported by Giannone et al. [10] Of note, the interobserver agreement in the standard 2D PACS group was within this previously reported range. Furthermore, median time needed by the participants to reach a conclusion was only 185 s (VR CT) and 116 s (VR MRI), representing a fast assessment. It is important to note that the study participants were free to use as much time to assess the scans as they liked, and interacted significantly shorter with the standard PACS imaging. The shorter viewing time in the 2D PACS viewing group may be attributed to the individuals’ familiarity with standard 2D assessment of scans. Participants are accustomed to this format which could lead to faster assessments. In contrast, 3D VR viewing invites the viewer to study the scans in more detail, using various tools at hand to freely interact with the scan from multiple angles. Increased exposure time could also be a part of the explanation for improved results in the 3D VR group, as participants take more time to study the scans before coming to a decision. To further assess the value of VR enhanced imaging to evaluate resectability of PDAC, a larger randomised prospective trial is needed, and correlation with perioperative as well as long-term clinical outcomes could shed light on the clinical value of this technology. Confirmation of these findings in a larger trial could support the transition of standard 2D PACS viewing to routine use of VR enhancement technology clinical practice.
As previously reported, interobserver agreement increases especially in cases of borderline resectability [20]. One of the main reasons contributing to this is that resectability represents a continuum rather than clear groups and might also be judged differently by individual surgeons. This issue has been recognised and recently a debate to redefine the terms ‘resectable’, ‘borderline resectable’ and ‘locally advanced’ has been proposed [10]. In our study cohort, comparison of interobserver agreement between the different resectability categories would require a larger study. Analysis of the variation between official MDT report and assessment of vascular infiltration and resectability status in VR reveals roughly three categories of errors. In the first category, imaging quality was insufficient or misleading, as in the case with the previous right hemicolectomy and the metal artefact. This scan was left in the final analysis to omit an imaging selection bias. The second category includes scans which allow for varying interpretations, such as the scan with the abutment of the SMA slightly below 180°. This scan was identified by one participant as R, but according to the guidelines and the MDT decision was seen as BR. The third category includes scans where the participant was not able to identify the tumour as such. Recently, sensitivity for CT and MRI to detect PDAC have been reported to be 75% and 70%, respectively [21]. Nonetheless, participants were able to identify PDAC and its resectability status overall rapidly and reliably, even surpassing the numbers reported in the literature. This is also taking into consideration that the participants were not accustomed to VR image viewing and have not had the chance to complete their learning curve when handling the VR system, although its use is largely intuitive.
A further added difficulty of resectability assessment based on imaging is the introduction of neoadjuvant chemotherapy in BR PDAC, as cross-sectional imaging appears to overestimate residual cancer and underestimate resectability [22]. Whether VR technology can improve assessment post-neoadjuvant chemotherapy could be a rewarding question for future studies in this context.
An interesting result from our study was that the pancreas was not identified on VR CT by all participants, which however did not represent an issue in the second study step. This is likely explained by the fact that also relatively inexperienced colleagues participated in the first study step, whereas only experienced surgeons and radiologists as well as surgeons who underwent subspecialist HPB training were included. Therefore, this result might reflect on the experience of reading cross-sectional imaging data rather than represent a weakness of the technology per se. In general, a lack of recognition of the tumour does not necessarily reflect a shortcoming of the technology but might be linked to the viewer’s experience and understanding. However, no correlation in the second study step was found between work experience and number of correct answers. Based on this finding, it can be assumed that clinicans can potentially use technology regardless of their experience. Of note, participants in this study preferred the use of VR CT over MRI, which could be explained by the fact CT imaging is widespread in daily clinical practice and surgeons might feel more confident interpreting these scans. However, the number of study participants is too low to draw a meaningful conclusion in this regard. Overall, however, there is controversy surrounding the value of MRI and CT for the assessment of PDAC, and it appears that MRI has a slight advantage over CT in terms of tumour detection rate, but CT appears to be superior for the assessment of vascular infiltration [23]. In this study, we have not included positron emission tomography (PET), and adding such scans to a future study could potentially increase the value of the VR system as tumours could be more easily identified.
The main limitations of this study are the relatively small number of participants in the second study step. Although VR enhanced CT imaging lead to better understanding of resectability of PDAC, a randomised prospective study, with a larger number of participants that directly compares VR to standard imaging and incorporates intraoperative outcomes, especially in BR cases, could offer further information on the clinical use of this software. A further future use of this technology could lie in patient education with the VR visualisation to demonstrate the imaging data to the patients, allowing for a better understanding of their disease and its treatment. Furthermore, implementing this technology in MDT could potentially yield effects such as enhanced collaboration among team members of different specialties, a more comprehensive analysis of medical imaging data and improved communication in case discussion. It is possible that the definitions of resectability will change in the future, and for this reason the implementation of a novel VR technology should not solely be based on the number of interobserver agreement, but rather also be based on clinical utility and user experience. In the future, connecting VR-enhanced visualisation data with AI algorithms that allow for automated assessment of vascular tumour contact might even revolutionise resectability assessment and preoperative planning [24, 25].
In conclusion, VR-enhanced CT and MRI visualisation is a promising tool to display abdominal anatomy that is easy for clinicians of varying levels of experience to interpret. Furthermore, there is the potential for VR-enhanced CT and MRI to be implemented as a tool to aid in future surgical planning and oncological treatment of PDAC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
Sieren JC, Ohno Y, Koyama H, Sugimura K, McLennan G (2010) Recent technological and application developments in computed tomography and magnetic resonance imaging for improved pulmonary nodule detection and lung cancer staging. J Magn Reson Imaging 32:1353–1369. https://doi.org/10.1002/jmri.22383
Shirk JD, Thiel DD, Wallen EM, Linehan JM, White WM, Badani KK, Porter JR (2019) Effect of 3-dimensional virtual reality models for surgical planning of robotic-assisted partial nephrectomy on surgical outcomes: a randomized clinical trial. JAMA Netw Open 2:e1911598–e1911598. https://doi.org/10.1001/jamanetworkopen.2019.11598
Maloca PM, Faludi B, Zelechowski M, Jud C, Vollmar T, Hug S, Müller PL, de Carvalho ER, Zarranz-Ventura J, Reich M, Lange C, Egan C, Tufail A, Hasler PW, Scholl HPN, Cattin PC (2020) Validation of virtual reality orbitometry bridges digital and physical worlds. Sci Rep 10:11815. https://doi.org/10.1038/s41598-020-68867-6
Maloca PM, de Carvalho JER, Heeren T, Hasler PW, Mushtaq F, Mon-Williams M, Scholl HPN, Balaskas K, Egan C, Tufail A, Witthauer L, Cattin PC (2018) High-performance virtual reality volume rendering of original optical coherence tomography point-cloud data enhanced with real-time ray casting. Transl Vis Sci Technol 7:2. https://doi.org/10.1167/tvst.7.4.2
Staubli SM, Maloca P, Kuemmerli C, Kunz J, Dirnberger AS, Allemann A, Gehweiler J, Soysal S, Droeser R, Däster S, Hess G, Raptis D, Kollmar O, von Flüe M, Bolli M, Cattin P (2022) Magnetic resonance cholangiopancreatography enhanced by virtual reality as a novel tool to improve the understanding of biliary anatomy and the teaching of surgical trainees. Front Surg 9:916443. https://doi.org/10.3389/fsurg.2022.916443
Sadeghi AH, Bakhuis W, Van Schaagen F, Oei FBS, Bekkers JA, Maat APWM, Mahtab EAF, Bogers AJJC, Taverne YJHJ (2020) Immersive 3D virtual reality imaging in planning minimally invasive and complex adult cardiac surgery. Eur Heart J - Digit Health 1:62–70. https://doi.org/10.1093/ehjdh/ztaa011
Hyde ER, Berger LU, Ramachandran N, Hughes-Hallett A, Pavithran NP, Tran MGB, Ourselin S, Bex A, Mumtaz FH (2019) Interactive virtual 3D models of renal cancer patient anatomies alter partial nephrectomy surgical planning decisions and increase surgeon confidence compared to volume-rendered images. Int J Comput Assist Radiol Surg 14:723–732. https://doi.org/10.1007/s11548-019-01913-5
Javaid M, Haleem A (2020) Virtual reality applications toward medical field. Clin Epidemiol Glob Health 8:600–605. https://doi.org/10.1016/j.cegh.2019.12.010
Zhao J, Xu X, Jiang H, Ding Y (2020) The effectiveness of virtual reality-based technology on anatomy teaching: a meta-analysis of randomized controlled studies. BMC Med Educ 20:127. https://doi.org/10.1186/s12909-020-1994-z
Giannone F, Capretti G, Abu Hilal M, Boggi U, Campra D, Cappelli C, Casadei R, De Luca R, Falconi M, Giannotti G, Gianotti L, Girelli R, Gollini P, Ippolito D, Limerutti G, Maganuco L, Malagnino V, Malleo G, Morone M, Mosconi C, Mrakic F, Palumbo D, Salvia R, Sgroi S, Zerbi A, Balzano G (2021) Resectability of pancreatic cancer is in the eye of the observer: a multicenter, blinded, prospective assessment of interobserver agreement on NCCN resectability status criteria. Ann Surg Open 2
Joo I, Lee JM, Lee ES, Son J-Y, Lee DH, Ahn SJ, Chang W, Lee SM, Kang H-J, Yang HK (2019) Preoperative CT classification of the resectability of pancreatic cancer: interobserver agreement. Radiology 293:343–349. https://doi.org/10.1148/radiol.2019190422
Tempero MA (2019) NCCN guidelines updates: pancreatic cancer. J Natl Compr Cancer Netw 17:603–605. https://doi.org/10.6004/jnccn.2019.5007
Faulkner L (2003) Beyond the five-user assumption: Benefits of increased sample sizes in usability testing. In: Behavior research methods, instruments, and computers
Dimitri Raptis rBiostatistics. In: rBiostatistics. https://www.rbiostatistics.com. Accessed 1 Jun 2023
Lin Q, Xu Z, Li B, Baucom R, Poulose B, Landman BA, Bodenheimer RE (2013) Immersive virtual reality for visualization of abdominal CT. In: Medical Imaging 2013: Image Perception, Observer Performance, and Technology Assessment
Das M, Sauer F, Schoepf UJ, Khamene A, Vogt SK, Schaller S, Kikinis R, vanSonnenberg E, Silverman SG (2006) Augmented reality visualization for CT-guided Interventions: system description, feasibility, and initial evaluation in an abdominal phantom. Radiology 240:230–235. https://doi.org/10.1148/radiol.2401040018
Birbara NS, Sammut C, Pather N (2020) Virtual reality in anatomy: a pilot study evaluating different delivery modalities. Anat Sci Educ 13:445–457. https://doi.org/10.1002/ase.1921
Liu J, Malekzadeh M, Mirian N, Song T-A, Liu C, Dutta J (2021) Artificial intelligence-based image enhancement in PET imaging: noise reduction and resolution enhancement. PET Clin 16:553–576. https://doi.org/10.1016/j.cpet.2021.06.005
Kim E, Shin G (2021) User discomfort while using a virtual reality headset as a personal viewing system for text-intensive office tasks. Ergonomics 64:891–899. https://doi.org/10.1080/00140139.2020.1869320
Caravatta L, Cellini F, Simoni N, Rosa C, Niespolo RM, Lupattelli M, Picardi V, Macchia G, Sainato A, Mantello G, Dionisi F, Rosetto ME, Fusco V, Navarria F, Paoli AD, Guido A, Vecchi C, Basilico R, Cianci R, Pizzi AD, Nicola MD, Mattiucci GC, Valentini V, Morganti AG, Genovesi D (2019) Magnetic resonance imaging (MRI) compared with computed tomography (CT) for interobserver agreement of gross tumor volume delineation in pancreatic cancer: a multi-institutional contouring study on behalf of the AIRO group for gastrointestinal cancers. Acta Oncol 58:439–447. https://doi.org/10.1080/0284186X.2018.1546899
Gorris M, Janssen QP, Besselink MG, Broek BLJ van den, Eijck CHJ van, Gils MJ van, Koerkamp BG, Struik F, Driel LMJW van, Hooft JE van (2022) Sensitivity of CT, MRI, and EUS-FNA/B in the preoperative workup of histologically proven left-sided pancreatic lesions. Pancreatology 22:136–141. https://doi.org/10.1016/j.pan.2021.11.008
Beleù A, Calabrese A, Rizzo G, Capelli P, Bellini N, Caloggero S, Calbi R, Tinazzi Martini P, De Robertis R, Carbognin G, Marchegiani G, Scarpa A, Salvia R, Bassi C, D’Onofrio M (2019) Preoperative imaging evaluation after downstaging of pancreatic ductal adenocarcinoma: a multi-center study. Cancers 11. https://doi.org/10.3390/cancers11020267
Chen F-M, Ni J-M, Zhang Z-Y, Zhang L, Li B, Jiang C-J (2016) Presurgical evaluation of pancreatic cancer: a comprehensive imaging comparison of CT versus MRI. Am J Roentgenol 206:526–535. https://doi.org/10.2214/AJR.15.15236
Anta JA, Martínez-Ballestero I, Eiroa D, García J, Rodríguez-Comas J (2022) Artificial intelligence for the detection of pancreatic lesions. Int J Comput Assist Radiol Surg 17:1855–1865. https://doi.org/10.1007/s11548-022-02706-z
Shimizu A, Kimoto T, Kobatake H, Nawano S, Shinozaki K (2010) Automated pancreas segmentation from three-dimensional contrast-enhanced computed tomography. Int J Comput Assist Radiol Surg 5:85–98. https://doi.org/10.1007/s11548-009-0384-0
Acknowledgements
The authors would like to thank Dimitri A. Raptis, MD, PhD, for making the rBiostatistics.com-platform available for free use.
Funding
Open access funding provided by University of Basel. This work was supported by the University of Basel, Switzerland (grant number 3MS1054 to SS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
P.C. Cattin is the inventor and owner of the VR application (Specto VR™) described in this study. The other authors have no conflicts of interest or financial ties to disclose.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The study was approved by the Ethics Committee of Northwest- and Central Switzerland (EKNZ). Approval-No: 2021–00457.
Informed consent
Informed consent was obtained from the patients before the study was conducted. Our institutions general informed consent was used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
About this article
Cite this article
Kunz, J.M., Maloca, P., Allemann, A. et al. Assessment of resectability of pancreatic cancer using novel immersive high-performance virtual reality rendering of abdominal computed tomography and magnetic resonance imaging. Int J CARS (2024). https://doi.org/10.1007/s11548-023-03048-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11548-023-03048-0