Abstract
Microvascular ultrasound (MVUS) is a new ultrasound technique that allows the detection of slow-velocity flow, providing the visualization of the blood flow in small vessels without the need of intravenous contrast agent administration. This technology has been integrated in the most recent ultrasound equipment and applied for the assessment of vascularization. Compared to conventional color Doppler and power Doppler imaging, MVUS provides higher capability to detect intralesional flow. A growing number of studies explored the potential applications in hepatobiliary, genitourinary, and vascular pathologies. Different flow patterns can be observed in hepatic and renal focal lesions providing information on tumor vascularity and improving the differential diagnosis. This article aims to provide a detailed review on the current evidences and applications of MVUS in abdominal imaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evaluation of vascular flow is an important part of ultrasound examination. Conventional Doppler techniques, such as color Doppler imaging (CDI) and power Doppler imaging (PDI), are commonly used to estimate vascularization of focal lesions. However, CDI and PDI have limited sensibility for the detection of slow vascular flow. Microvascular ultrasound (MVUS) is a new ultrasound technique that allows the detection of slow-velocity flow, providing the visualization of the blood flow in small vessels without the need of intravenous contrast agent administration [1, 2]. Recently, MVUS techniques have been developed by different vendors and integrated in the ultrasound equipment for clinical use, as provided in Table 1.
Detailed description of the MVUS has been provided in prior publications [3,4,5]. Briefly, CDI and PDI techniques eliminate artifacts caused by tissue movements and clutter by applying a monodimensional wall filter, which also removes the slow blood flow signals that occupy the same bandwidth on the frequency domain [4]. The microvascular technology applies an advanced filter to separate the slow flow signal from the clutter signal [5]. This allows to preserve the slow flow signal originating from microvasculature. The corresponding flow information can be displayed in color mode on conventional gray-scale ultrasound images with embedded color-encoded flow signals or as monochrome mode which focuses on the vascular signal only and further enhances the vascular pattern by suppressing the background signal [3]. The superb microvascular imaging allows to calculate the vascular index, which is a quantitative parameter representing the percentage of color pixels on the total pixels number within a region of interest [3, 5]. It can be calculated with a dedicated application of the US device by placing a region of interest with standardized size or by drawing the contour of the area of interest [5]. Finally, it should be noted that the application of MVUS in abdominal imaging can be limited by the lesion depth with microvascular flow being less detectable in deeper regions, smaller box size compared to other Doppler techniques, and large motion artifacts [1].
The MVUS have been widely applied for the diagnosis of thyroid pathologies and breast lesions [6, 7]. Recently, several abdominal applications have been explored to improve the diagnosis of focal and diffuse abdominal pathologies (Table 2). This article aims to provide an up-to-date review on the current evidences and applications of MVUS in abdominal imaging.
Hepatic applications
Vascularization of hepatic lesions with MVUS was analyzed by several studies, and different vascular patterns were associated with specific lesions [8, 9]. In an initial study on 29 liver lesions, Lee et al. [10] reported nodular rim pattern and spotty dot-like pattern in 33% and 20% of hemangiomas, respectively, while focal nodular hyperplasia (FNH) exhibited more commonly a spoke-wheel (43%) or radiating (29%) pattern. Dubinsky et al. [11] and Han et al. [12] identified a higher number of central and peripheral vessels in hepatocellular carcinomas (HCCs) compared to benign lesions by using MVUS. In a study including 92 hemangiomas, the most common vascular patterns detected by superb microvascular imaging were the strip rim (48.4%) and the peripheral nodular rim (37.1%) patterns (Fig. 1) [13]. However, about one-third of the hemangiomas did not show any vascular signal, more frequently being lesions smaller than 2 cm [13]. In a recent prospective study, Jeon and colleagues [14] demonstrated a significantly higher sensitivity for the vascular flow detection and higher tumor vascularity score with MVUS compared to CDI and PDI. In this study, hemangiomas demonstrated more commonly a nodular rim pattern (52.2%) or strip rim pattern (26.1%), and FNHs were associated with a spoke-wheel pattern (66.7%), while malignant tumors had an irregular nonspecific vascular pattern (66.7%) [14]. The irregular vascular pattern (Fig. 2) was the most commonly reported pattern in HCCs by Yang et al. [15], while Bae et al. [16] described the basket pattern in HCC characterized by a combination of peripheral rim of vascularization and irregular internal vessels. Recent studies reported a significant correlation between the quantification of vascular index and the microvessel density at the histopathological analysis in HCCs and hepatic metastases [15, 17]. Moreover, Kang et al. [18] prospectively evaluated 100 patients with HCC treated with transarterial chemoembolization. In this study microflow imaging had a sensitivity of 79.3% and a specificity of 80% for the detection of residual intratumoral flow [18].
40-year-old woman with a 5.7 cm hepatic hemangioma incidentally detected on abdominal ultrasound examination. Color (a) and directional power Doppler (b) imaging demonstrate minimal peripheral vascularization. Microvascular flow imaging shows a strip rim pattern of vascularization (c). Contrast-enhanced ultrasound (CEUS) confirmed the diagnosis of hepatic hemangioma by showing the peripheral globular enhancement (d, 63 s after contrast injection)
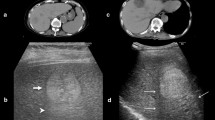
78-year-old man with hepatocellular carcinoma. B-mode ultrasound (a) shows a 2.0 cm hypoechoic lesion in the right hepatic lobe (arrow). Color (b) and power Doppler (c) imaging demonstrate mild peripheral vascularization. Microvascular imaging clearly depicts both peripheral and irregular intratumoral vascular pattern (d)
The different vascular patterns may be explained by the different histopathological tumoral features. Hemangiomas were associated with a peripheral strip rim pattern or nodular rim pattern with dot-like spots, which can be related to the dilated peripheral vascular spaces. The spoke-wheel pattern of FNH is related to its internal vascular architecture, with intralesional arteries radiating from the center toward the periphery of the lesion [19, 20]. Peripheral draining veins can also be visualized in FNH [21]. The irregular vascular pattern of malignant tumors and HCCs reflects the abnormal tumoral neoangiogenesis, leading to the development of irregular intralesional vessels.
Ultrasound evaluation of the nonlesional liver parenchyma is also performed to assess morphological changes associated with advanced liver disease and detection of hepatic steatosis. Liver biopsy is the reference standard for the fibrosis staging, but it is an invasive procedure that carries risks of complications and sampling errors. Therefore, several advanced imaging methods have been evaluated over time as potential noninvasive tools for the fibrosis staging [22,23,24]. Vascular changes and distortions, as result of progressive fibrosis accumulation, were explored with MVUS [25, 26]. A study by Balik et al. [27] identified subcapsular small vessels blunting on superb microvascular imaging as a relevant finding for the prediction of hepatic fibrosis. In the study by Tosun and colleagues [28], the vascular score obtained with the superb microvascular imaging significantly correlated with the liver fibrosis stage at histopathology. Interestingly, in that study the vascular score predicted fibrosis with higher accuracy compared to the SWE [28].
Furthermore, Gao et al. [29] correlated the vascular index obtained with superb microvascular imaging with the presence of hepatic steatosis using the proton density fat fraction as reference standard. In that study, patients with hepatic steatosis had a significantly lower vascular index compared to normal controls, likely reflecting the changes in microvascularity due to fat accumulation within the hepatocytes [29].
MVUS was also employed to improve the assessment of the hepatic artery in posttransplant evaluation, showing improved visibility compared to CDI; therefore, MVUS could be used to improve the diagnosis of hepatic artery stenosis and thrombosis in transplanted patients [30,31,32]. In an initial experience by Jang and colleagues [30] on 56 patients, superb microvascular imaging improved the visibility of hepatic artery compared to CDI and provided a good-to-excellent inter-reader reproducibility for hepatic artery measurements. Similarly, Güven et al. [31] reported that superb microvascular imaging allowed to visualize vascular flow in the hepatic artery in 16/20 patients in which normal flow was undetectable on CDI. In pediatric liver transplantation, the hepatic artery visibility score was significantly higher in superb microvascular imaging compared to CDI [32]. In a study by Gu et al. [33], the MVUS provided a sensitivity of 100% and a specificity of 98.9% for the diagnosis of hepatic artery thrombosis in 105 transplanted children, which was similar to CDI (sensitivity of 100% and specificity of 92.8%).
Gallbladder, pancreatic and splenic applications
Gallbladder
In patients with suspected acute cholecystitis, the quantitative assessment with the superb microvascular imaging allowed to measure the hyperemic changes in the gallbladder bed, improving the diagnosis of acute cholecystitis [34]. Correlation with the severity of inflammation and complications of acute cholecystitis need to be further explored [35, 36]. An initial study including 20 patients also evaluated the feasibility of contrast-enhanced superb microvascular imaging for the vascular evaluation of gallbladder lesions (Fig. 3) demonstrating a significantly higher frequency of tortuous microvessels and abrupt caliber change in malignancies comparted to benign gallbladder lesions [37].
49-year-old woman with incidentally detected 1.2 cm gallbladder polyp. Color (a), directional power Doppler (b), and microvascular flow imaging (c) demonstrate intralesional vascular signal, with branching appearance on MVUS (arrow). The lesion shows homogeneous enhancement on contrast-enhanced ultrasound at 32 s after contrast injection (d). This lesion was confirmed as an adenomatous polyp after cholecystectomy
Pancreas and spleen
Currently, there is a lack of studies supporting the use of MVUS in pancreatic and splenic lesions, with only few published case reports [38, 39]. Particularly, Tokodai et al. [38] applied superb microvascular imaging to monitor splenic vein patency in a patient undergoing pancreatic transplantation. Yamanaka et al. [39] described the microvascular flow findings in two patients with splenic artery pseudoaneurysm. In both cases, MVUS better depicted vascular flow compared to other Doppler techniques. Future studies are needed to explore the potential applications of MVUS in pancreas and spleen.
Genitourinary applications
Kidneys
MVUS has the advantage to provide higher sensitivity for the assessment of renal microvasculature compared to conventional Doppler techniques [40]. Improved detection of intralesional vascularization could be particularly helpful in the differential diagnosis of benign and malignant renal tumors. Mao et al. [41] firstly assessed the use of MVUS in 53 patients with solid renal tumors, demonstrating significantly higher vascularity in malignant tumors by using the superb microvascular imaging, with an annular blood flow pattern being more commonly detected in malignant tumors (67% vs 9% of benign tumors). Similarly, Leong et al. [42] showed that superb microvascular imaging had the highest sensitivity in detecting tumor vascularity compared to conventional Doppler techniques in 50 indeterminate renal lesions. Chen and colleagues [43] analyzed 63 patients with solid renal tumors with different Doppler techniques. In this study, ring-like blood flow was detected in 37/57 malignant tumors by superb microvascular imaging, which provided a sensitivity of 82.4% and a specificity of 88.8% for the differential diagnosis between benign and malignant lesions [43]. This pattern of vascularization likely correlates with the presence of a pseudocapsule in renal cell carcinoma with fibrotic tissue and capillary vessels. In a large study including 144 solid renal lesions, the intratumoral flow detection rate was reported to be 78.5% with CDI, 88.9% with MVUS, and 93.8% with contrast-enhanced MVUS [44]. Furthermore, superb microvascular imaging demonstrated higher accuracy compared to CDI for the diagnosis of malignancy according to the Bosniak classification in patients with cystic renal lesions (Fig. 4) with improved detection of microvascular flow in cystic septa [45].
56-year-old man with 5 cm cystic renal lesion detected on computed tomography. Color (a), directional power doppler (b), and microvascular flow imaging (c) do not detect any intracystic vascularization. Contrast-enhanced ultrasound (d) shows septa with minimal enhancement at 58 s after contrast injection. The lesion was classified as bosniak IIF
MVUS also provided higher detectability of acute pyelonephritis compared to conventional B-mode ultrasound and CDI, with improved detection of hypoperfused cortical areas [46]. Finally, the cortical microvascular flow and vascular index were correlated with the renal function, and lower flow values were associated with the development and severity of chronic kidney disease [47].
In patients receiving kidney transplantation, MVUS showed a decrease of cortical microvasculature with the progressive deterioration of renal function [48]. A recently published study correlated the capsule-to-vessels distance in transplanted kidneys with the chronic allograft damage index, with similar performance of MVUS compared to other Doppler techniques [49].
Bladder
Few studies investigated the potential applications of MVUS in the urinary bladder (Fig. 5). Ates and coworkers [50] measured the vascular index in the anterior bladder wall to diagnose acute cystitis in pediatric patients with a reported sensitivity of 93% and specificity of 92% for the diagnosis of acute cystitis. Kim et al. [51] applied the superb microvascular imaging in the detection of vesicoureteral reflux, which allowed to demonstrate reversed ureteral jet or renal pelvic swirl sign in 75% of patients.
64-year-old man with bladder cancer. Ultrasound examination with microvascular flow imaging (a) shows a 2.3 cm lesion of the bladder wall with intralesional vascularization (arrow). The lesion demonstrates strong enhancement on contrast-enhanced ultrasound at 50 s after contrast injection (b, arrow)
Prostate
Transrectal ultrasound-guided biopsy is widely performed to histopathologically diagnose prostate cancer. Zhu et al. [52] analyzed 119 patients who underwent transrectal ultrasound before biopsy. MVUS was able to detect microvascularity in 97.3% of prostatic cancers, and it significantly correlated with the Gleason score [52]. Shen et al. [53] demonstrated that target biopsy guided by the superb microvascular imaging allowed to obtain a higher rate of prostate cancer detection than systematic biopsy. If this data will be confirmed by further studies, MVUS may provide a promising role in guiding target biopsy on transrectal ultrasound.
Gynecological and obstetric applications
MVUS has been applied to diagnose gynecological and obstetrical conditions in multiple studies. Assessment of vascularization of the ovaries is an important finding in gynecological ultrasound examinations. MVUS demonstrated improved visibility of ovarian vascularity compared to conventional Doppler techniques in healthy patients [54]. In patients with uterine fibroids, Samanci et al. [55] evaluated the role of MVUS for the prediction of response in patients undergoing uterine artery embolization, observing that a higher preoperative vascularization was associated with higher volume reduction.
In pregnant women, MVUS was applied to evaluate placental vascularization and vessels [56,57,58]. This may be helpful to detect placental abnormalities with higher sensitivity, particularly alterations of placenta attachment or placenta accreta identification [59,60,61]. Other applications in obstetrics explored the visualization of fetal structures and vasculature such as brain and intra-abdominal vessels [62, 63]. In the initial experience reported by Hata and colleagues [63] superb microvascular imaging was able to visualize abdominal organ microvasculature in the majority of normal fetus at 22–40 weeks of gestation.
Vascular applications
In patients undergoing endovascular aneurysm repair (EVAR), ultrasound examination can be performed to monitor the aneurysmal sac size and detect the presence of endoleak, which is characterized by persistent blood flow in the aneurysmal sac [64, 65]. The effectiveness of MVUS for the diagnosis of endoleak (Fig. 6) was explored in recent studies [66,67,68]. A study by Cantisani et al. [66] firstly demonstrated that MVUS had higher accuracy (63% sensitivity and 96% specificity) for the detection of endoleak compared to CDI in 57 patients treated with EVAR, although the performance was lower compared to CEUS and CT angiography. Gabriel et al. [67] reported the same accuracy of MVUS and CEUS for the detection of endoleak (sensitivity 100%, specificity 93%, accuracy 97%) in 30 patients followed-up after EVAR. Similarly, a recent study by Curti and colleagues [68] reported the same sensitivity (91.5%) and specificity (100%) of MVUS and CEUS for the detection of type II endoleak in 122 patients.
76-year-old man undergoing follow-up examination after endovascular aneurysm repair. Ultrasound examination with color doppler imaging (a) shows patency of the graft without signs of endoleak. Microvascular flow imaging (b) demonstrates the presence of a peripherally located endoleak (arrow), consistent with type II endoleak from a lumbar artery, which was confirmed on contrast-enhanced ultrasound (c, arrow, at 96 s after contrast injection)
Finally, MVUS has the potential to visualize vascularization in the atherosclerotic plaques, which have an increased risk of complications, but studies on abdominal vessels are still missing [69].
Conclusion
Microvascular ultrasound imaging improves the detection of vascularization compared to color and power Doppler imaging. The integration of MVUS technology in the new ultrasound equipment provides to radiologists a rapid and versatile tool for the multiparametric ultrasound assessment of various abdominal conditions. In clinical practice, new ultrasound microvascular techniques can be used in conjunct with traditional Doppler imaging to improve the diagnostic performance and detection of vascularity. The vascular patterns detected in hepatic and renal focal lesions have the potential to increase the confidence toward a diagnosis of malignancy or benignity in noncontrast ultrasound examination.
References
Aziz MU, Eisenbrey JR, Deganello A, Zahid M, Sharbidre K, Sidhu P, Robbin ML (2022) Microvascular flow imaging: a state-of-the-art review of clinical use and promise. Radiology 305:250–264. https://doi.org/10.1148/radiol.213303
Bartolotta TV, Taibbi A, Randazzo A, Gagliardo C (2021) New frontiers in liver ultrasound: from mono to multi parametricity. World J Gastrointest Oncol 13:1302–1316. https://doi.org/10.4251/wjgo.v13.i10.1302
Park AY, Seo BK (2018) Up-to-date doppler techniques for breast tumor vascularity: superb microvascular imaging and contrast-enhanced ultrasound. Ultrasonography 37:98–106. https://doi.org/10.14366/usg.17043
Jiang ZZ, Huang YH, Shen HL, Liu XT (2019) Clinical applications of superb microvascular imaging in the liver, breast, thyroid, skeletal muscle, and carotid plaques. J Ultrasound Med 38:2811–2820. https://doi.org/10.1002/jum.15008
Tang K, Liu M, Zhu Y, Zhang M, Niu C (2022) The clinical application of ultrasonography with superb microvascular imaging-a review. J Clin Ultrasound 50:721–732. https://doi.org/10.1002/jcu.23210
Fu Z, Zhang J, Lu Y, Wang S, Mo X, He Y, Wang C, Chen H (2021) Clinical applications of superb microvascular imaging in the superficial tissues and organs: a systematic review. Acad Radiol 28:694–703. https://doi.org/10.1016/j.acra.2020.03.032
Bartolotta TV, Orlando AAM, Schillaci MI, Spatafora L, Marco MD, Matranga D, Firenze A, Cirino A, Ienzi R (2021) Ultrasonographic detection of vascularity of focal breast lesions: microvascular imaging versus conventional color and power doppler imaging. Ultrason Imaging 43:273–281. https://doi.org/10.1177/01617346211029542
Wilson A, Lim AKP (2022) Microvascular imaging: new doppler technology for assessing focal liver lesions. Is it useful? Clin Radiol 77:e807–e820. https://doi.org/10.1016/j.crad.2022.05.032
He MN, Lv K, Jiang YX, Jiang TA (2017) Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol 23:7765–7775. https://doi.org/10.3748/wjg.v23.i43.7765
Lee DH, Lee JY, Han JK (2016) Superb microvascular imaging technology for ultrasound examinations: Initial experiences for hepatic tumors. Eur J Radiol 85:2090–2095. https://doi.org/10.1016/j.ejrad.2016.09.026
Dubinsky TJ, Revels J, Wang S, Toia G, Sonneborn R, Hippe DS, Erpelding T (2018) Comparison of superb microvascular imaging with color flow and power doppler imaging of small hepatocellular carcinomas. J Ultrasound Med 37:2915–2924. https://doi.org/10.1002/jum.14654
Han H, Ji Z, Ding H, Zhang W, Zhang R, Wang W (2019) Assessment of blood flow in the hepatic tumors using non-contrast micro flow imaging: Initial experience. Clin Hemorheol Microcirc 73:307–316. https://doi.org/10.3233/CH-180532
Jeon SK, Lee JY, Han JK (2021) Superb microvascular imaging technology of ultrasound examinations for the evaluation of tumor vascularity in hepatic hemangiomas. Ultrasonography 40:538–545. https://doi.org/10.14366/usg.20177
Jeon SK, Lee JY, Kang HJ, Han JK (2022) Additional value of superb microvascular imaging of ultrasound examinations to evaluate focal liver lesions. Eur J Radiol 152:110332. https://doi.org/10.1016/j.ejrad.2022.110332
Yang F, Zhao J, Liu C, Mao Y, Mu J, Wei X, Jia J, Zhang S, Xin X, Tan J (2019) Superb microvascular imaging technique in depicting vascularity in focal liver lesions: more hypervascular supply patterns were depicted in hepatocellular carcinoma. Cancer Imaging 19:92. https://doi.org/10.1186/s40644-019-0277-6
Bae JS, Lee JM, Jeon SK, Jang S (2020) Comparison of microflow imaging with color and power doppler imaging for detecting and characterizing blood flow signals in hepatocellular carcinoma. Ultrasonography 39:85–93. https://doi.org/10.14366/usg.19033
Kratzer W, Güthle M, Dobler F, Seufferlein T, Graeter T, Schmidberger J, Barth TF, Klaus J (2022) Comparison of superb microvascular imaging (SMI) quantified with ImageJ to quantified contrast-enhanced ultrasound (qCEUS) in liver metastases-a pilot study. Quant Imaging Med Surg 12:1762–1774. https://doi.org/10.21037/qims-21-383
Kang HJ, Lee JM, Jeon SK, Ryu H, Yoo J, Lee JK, Han JK (2019) Microvascular flow imaging of residual or recurrent hepatocellular carcinoma after transarterial chemoembolization: comparison with color/power doppler imaging. Korean J Radiol 20:1114–1123. https://doi.org/10.3348/kjr.2018.0932
Bonacchi G, Becciolini M, Seghieri M (2017) Superb microvascular imaging: a potential tool in the detection of FNH. J Ultrasound 20:179–180. https://doi.org/10.1007/s40477-017-0240-y
Rónaszéki AD, Dudás I, Zsély B, Budai BK, Stollmayer R, Hahn O, Csongrády B, Park BS, Maurovich-Horvat P, Győri G, Kaposi PN (2023) Microvascular flow imaging to differentiate focal hepatic lesions: the spoke-wheel pattern as a specific sign of focal nodular hyperplasia. Ultrasonography 42:172–181. https://doi.org/10.14366/usg.22028
Naganuma H, Ishida H, Ogawa M (2001) Suzuki K (2017) Visualization of draining vein in focal nodular hyperplasia by superb microvascular imaging: report of two cases. J Med Ultrason 44:323–328. https://doi.org/10.1007/s10396-017-0775-8
Vernuccio F, Cannella R, Bartolotta TV, Galia M, Tang A, Brancatelli G (2021) Advances in liver US, CT, and MRI: moving toward the future. Eur Radiol Exp 5:52. https://doi.org/10.1186/s41747-021-00250-0
Petitclerc L, Sebastiani G, Gilbert G, Cloutier G, Tang A (2017) Liver fibrosis: review of current imaging and MRI quantification techniques. J Magn Reson Imaging 45:1276–1295. https://doi.org/10.1002/jmri.25550
Taibbi A, Petta S, Matranga D, Caruana G, Cannella R, Busè G, Marco VD, Midiri M, Bartolotta TV (2021) Liver stiffness quantification in biopsy-proven nonalcoholic fatty liver disease patients using shear wave elastography in comparison with transient elastography. Ultrasonography 40:407–416. https://doi.org/10.14366/usg.20147
Kuroda H, Abe T, Kakisaka K, Fujiwara Y, Yoshida Y, Miyasaka A, Ishida K, Ishida H, Sugai T, Takikawa Y (2016) Visualizing the hepatic vascular architecture using superb microvascular imaging in patients with hepatitis C virus: A novel technique. World J Gastroenterol 22:6057–6064. https://doi.org/10.3748/wjg.v22.i26.6057
Koyama N, Hata J, Sato T, Tomiyama Y, Hino K (2017) Assessment of hepatic fibrosis with superb microvascular imaging in hepatitis C virus-associated chronic liver diseases. Hepatol Res 47:593–597. https://doi.org/10.1111/hepr.12776
Balık AÖ, Kılıçoğlu ZG, Görmez A, Özkara S (2019) Radiology-pathology correlation in staging of liver fibrosis using superb microvascular imaging. Diagn Interv Radiol 25:331–337. https://doi.org/10.5152/dir.2019.18231
Tosun M, Uslu H (2022) Comparison of superb microvascular imaging and shear wave elastography for assessing liver fibrosis in chronic hepatitis B. Ultrasonography 41:394–402. https://doi.org/10.14366/usg.21136
Gao J, King J, Chatterji M, Miller BR, Siddoway RL (2022) Superb microvascular imaging-based vascular index to assess adult hepatic steatosis: a feasibility study. Ultrasound Med Biol 48:480–487. https://doi.org/10.1016/j.ultrasmedbio.2021.11.002
Jang HY, Kim KW, Kim SY, Kim JS, Choi SH, Kim SY, Lee SG (2018) Visibility of the graft hepatic artery using superb microvascular imaging in liver transplantation recipients: initial experience. Acta Radiol 59:1326–1335. https://doi.org/10.1177/0284185118757275
Güven F, Karaca L, Ogul H, Sade R, Öztürk G, Kantarci M (2019) The value of superb microvascular imaging in detecting hepatic artery occlusion in liver transplantation: a preliminary study. Ultrasound Q 35:325–329. https://doi.org/10.1097/RUQ.0000000000000416
Collaku E, Simonini R, Balbi M, Bonaffini PA, Valle C, Morzenti C, Faseli RF, Ferrari A, Ippolito D, Marra P, Barbui T, Sironi S (2022) Superb microvascular imaging (SMI) compared with color doppler ultrasound for the assessment of hepatic artery in pediatric liver transplants: a feasibility study. Diagnostics (Basel) 12:1476. https://doi.org/10.3390/diagnostics12061476
Gu LH, Fang H, Liu XS, Xia Q, Li FH (2020) Additional value of superb microvascular imaging for assessing hepatic arterial blood flow after pediatric liver transplantation. Pediatr Transplant 24:e13785. https://doi.org/10.1111/petr.13785
Ra JC, Lee ES, Park HJ, Kim HS, Lee JB, Do JH, Park SB, Choi BI (2018) Efficacy of superb microvascular imaging for diagnosing acute cholecystitis: comparison with conventional ultrasonography. Ultrasound Med Biol 44:1968–1977. https://doi.org/10.1016/j.ultrasmedbio.2018.05.014
Tomizawa M, Shinozaki F, Motoyoshi Y, Sugiyama T, Yamamoto S, Ishige N (2016) Signal intensity of superb microvascular imaging correlates with the severity of acute cholecystitis. Case Rep Gastroenterol 10:452–458. https://doi.org/10.1159/000446765
Aziz MU, Robbin ML (2022) Improved detection of gallbladder perforation using ultrasound small vessel slow flow “perfusion” imaging. J Ultrasound Med 41:511–518. https://doi.org/10.1002/jum.15729
Kin T, Nagai K, Hayashi T, Takahashi K, Katanuma A (2020) Efficacy of superb microvascular imaging of ultrasound for diagnosis of gallbladder lesion. J Hepatobiliary Pancreat Sci 27:977–983. https://doi.org/10.1002/jhbp.841
Tokodai K, Miyagi S, Nakanishi C, Hara Y, Nakanishi W, Miyazawa K, Shimizu K, Goto M, Kamei T (2001) Unno M (2018) The utility of superb microvascular imaging for monitoring low-velocity venous flow following pancreas transplantation: report of a case. J Med Ultrason 45:171–174. https://doi.org/10.1007/s10396-017-0795-4
Yamanaka Y, Ishida H, Naganuma H, Komatsuda T, Miyazawa H, Miyauchi T, Takahashi S, Tozawa T, Enomoto K (2018) Superb microvascular imaging (SMI) findings of splenic artery pseudoaneurysm: a report of two cases. J Med Ultrason 45:515–523. https://doi.org/10.1007/s10396-018-0858-1
Gao J, Thai A, Erpelding T (2019) Comparison of superb microvascular imaging to conventional color doppler ultrasonography in depicting renal cortical microvasculature. Clin Imaging 58:90–95. https://doi.org/10.1016/j.clinimag.2019.06.011
Mao Y, Mu J, Zhao J, Zhao L, Xin X (2018) The value of superb microvascular imaging in differentiating benign renal mass from malignant renal tumor: a retrospective study. Br J Radiol 91:20170601. https://doi.org/10.1259/bjr.20170601
Leong JY, Wessner CE, Kramer MR, Forsberg F, Halpern EJ, Lyshchik A, Torkzaban M, Morris A, Byrne K, VanMeter M, Trabulsi EJ, Lallas CD, Eisenbrey JR (2020) Superb microvascular imaging improves detection of vascularity in indeterminate renal masses. J Ultrasound Med 39:1947–1955. https://doi.org/10.1002/jum.15299
Chen M, Fu X, Shen Y (2021) Evaluation of multimode color doppler flow imaging in the diagnosis of solid renal tumor. Contrast Media Mol Imaging 2021:6656877. https://doi.org/10.1155/2021/6656877
Mao Y, Mu J, Zhao J, Yang F, Zhao L (2022) The comparative study of color doppler flow imaging, superb microvascular imaging, contrast-enhanced ultrasound micro flow imaging in blood flow analysis of solid renal mass. Cancer Imaging 22:21. https://doi.org/10.1186/s40644-022-00458-2
Mu J, Mao Y, Li F, Xin X, Zhang S (2019) Superb microvascular imaging is a rational choice for accurate Bosniak classification of renal cystic masses. Br J Radiol 92:20181038. https://doi.org/10.1259/bjr.20181038
Choi G, Je BK, Hong D, Cha J (2021) Microvascular doppler ultrasound in children with acute pyelonephritis. Med Ultrason 23:161–167. https://doi.org/10.11152/mu-2827
Armaly Z, Abu-Rahme M, Kinaneh S, Hijazi B, Habbasshi N, Artul S (2022) An innovative ultrasound technique for early detection of kidney dysfunction: superb microvascular imaging as a reference standard. J Clin Med 11:925. https://doi.org/10.3390/jcm11040925
Marticorena Garcia SR, Guo J, Dürr M, Denecke T, Hamm B, Sack I, Fischer T (2018) Comparison of ultrasound shear wave elastography with magnetic resonance elastography and renal microvascular flow in the assessment of chronic renal allograft dysfunction. Acta Radiol 59:1139–1145. https://doi.org/10.1177/0284185117748488
Gürbüz AF, Keven A, Elmalı A, Toru S, Apaydın A, Çeken K (2023) A comparison between the superb microvascular imaging technique and conventional doppler ultrasound in evaluating chronic allograft damage in renal transplant recipients. Diagn Interv Radiol 29:212–218. https://doi.org/10.5152/dir.2022.21555
Ates F, Durmaz MS, Yorulmaz A, Sara HI (2022) Quantitative assessment of bladder wall vascularity index in children with acute cystitis using superb microvascular imaging. J Ultrasound 25:27–33. https://doi.org/10.1007/s40477-020-00549-5
Kim HK, O’Hara S, Je BK, Kraus SJ, Horn P (2018) Feasibility of superb microvascular imaging to detect high-grade vesicoureteral reflux in children with urinary tract infection. Eur Radiol 28:66–73. https://doi.org/10.1007/s00330-017-4974-x
Zhu YC, Shan J, Zhang Y, Jiang Q, Wang YB, Deng SH, Qu QH, Li Q (2019) Prostate cancer vascularity: superb microvascular imaging ultrasonography with histopathology correlation. Med Sci Monit 25:8571–8578. https://doi.org/10.12659/MSM.918318
Shen TT, Xue JL (2019) Impact of a novel ultrasound microvascular imaging and elastography on prostate cancer classification. Transl Androl Urol 8:696–702. https://doi.org/10.21037/tau.2019.11.15
Ayaz E, Aslan A, İnan İ, Yıkılmaz A (2019) Evaluation of ovarian vascularity in children by using the “superb microvascular imaging” ultrasound technique in comparison with conventional doppler ultrasound techniques. J Ultrasound Med 38:2751–2760. https://doi.org/10.1002/jum.14983
Samanci C, Ozkose B, Ustabasioglu FE, Erol BC, Sirolu S, Yılmaz F, Ozkose ZG, Yılmaz H, Kara SC, Kicik Caliskan R, Gulsen F (2021) The diagnostic value of superb microvascular imaging in prediction of uterine artery embolization treatment response in uterine leiomyomas. J Ultrasound Med 40:2607–2615. https://doi.org/10.1002/jum.15647
Hasegawa J, Suzuki N (2016) SMI for imaging of placental infarction. Placenta 47:96–98. https://doi.org/10.1016/j.placenta.2016.08.092
Mack LM, Mastrobattista JM, Gandhi R, Castro EC, Burgess APH, Lee W (2019) Characterization of placental microvasculature using superb microvascular imaging. J Ultrasound Med 38:2485–2491. https://doi.org/10.1002/jum.14919
Sun L, Li N, Jia L, Zhang C, Wang S, Jiao R, Wang L, Ye Y (2020) comparison of superb microvascular imaging and conventional doppler imaging techniques for evaluating placental microcirculation: a prospective study. Med Sci Monit 26:e926215. https://doi.org/10.12659/MSM.926215
Hasegawa J, Kurasaki A, Hata T, Homma C, Miura A, Kondo H, Suzuki N (2019) Diagnosis of placenta accreta spectrum using ultra-high-frequency probe and superb microvascular imaging. Ultrasound Obstet Gynecol 54:705–707. https://doi.org/10.1002/uog.20207
Chen X, Wei X, Zhao S, Huang H, Wang W, Qiu J, Chen X, Cheng C, Tian Z, Rychik J (2021) Characterization of placental microvascular architecture by MV-flow imaging in normal and fetal growth-restricted pregnancies. J Ultrasound Med 40:1533–1542. https://doi.org/10.1002/jum.15531
Horinouchi T, Yoshizato T, Kojiro-Sanada S, Kozuma Y, Yokomine M, Ushijima K (2021) Missing decidual doppler signals as a new diagnostic criterion for placenta accreta spectrum: a case described using superb microvascular imaging. J Obstet Gynaecol Res 47:411–415. https://doi.org/10.1111/jog.14441
Hasegawa J, Yamada H, Kawasaki E, Matsumoto T, Takahashi S, Suzuki N (2018) Application of superb micro-vascular imaging (SMI) in obstetrics. J Matern Fetal Neonatal Med 31:261–263. https://doi.org/10.1080/14767058.2016.1278206
Hata T, Koyanagi A, Yamanishi T, Bouno S, Takayoshi R, Miyake T (2020) Superb microvascular imaging with doppler luminance using an 18-MHz probe to visualize fetal intra-abdominal blood vessels and organ microvasculature. J Perinat Med 48:184–188. https://doi.org/10.1515/jpm-2019-0411
Smith T, Quencer KB (2020) Best practice guidelines: imaging surveillance after endovascular aneurysm repair. AJR Am J Roentgenol 214:1165–1174. https://doi.org/10.2214/AJR.19.22197
Gozzo C, Caruana G, Cannella R, Farina A, Giambelluca D, Dinoto E, Vernuccio F, Basile A, Midiri M (2022) CT angiography for the assessment of EVAR complications: a pictorial review. Insights Imaging 13:5. https://doi.org/10.1186/s13244-021-01112-4
Cantisani V, David E, Ferrari D, Fanelli F, Di Marzo L, Catalano C, Benedetto F, Spinelli D, Katsargyris A, Blandino A, Ascenti G, D’Ambrosio F (2017) Color doppler ultrasound with superb microvascular imaging compared to contrast-enhanced ultrasound and computed tomography angiography to identify and classify endoleaks in patients undergoing EVAR. Ann Vasc Surg 40:136–145. https://doi.org/10.1016/j.avsg.2016.06.038
Gabriel M, Tomczak J, Snoch-Ziółkiewicz M, Dzieciuchowicz Ł, Strauss E, Pawlaczyk K, Wojtusik D, Oszkinis G (2018) Superb micro-vascular Imaging (SMI): a doppler ultrasound technique with potential to identify, classify, and follow up endoleaks in patients after endovascular aneurysm repair (EVAR). Abdom Radiol (NY) 43:3479–3486. https://doi.org/10.1007/s00261-018-1633-x
Curti M, Piacentino F, Fontana F, Ossola C, Coppola A, Marra P, Basile A, Ierardi AM, Carrafiello G, Carcano G, Tozzi M, Piffaretti G, Venturini M (2022) EVAR follow-up with ultrasound superb microvascular imaging (SMI) compared to CEUS and CT angiography for detection of type II endoleak. Diagnostics (Basel) 12(2):526. https://doi.org/10.3390/diagnostics12020526
Zamani M, Skagen K, Scott H, Lindberg B, Russell D, Skjelland M (2019) Carotid plaque neovascularization detected with superb microvascular imaging ultrasound without using contrast media. Stroke 50:3121–3127. https://doi.org/10.1161/STROKEAHA.119.025496
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by RC and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Roberto Cannella has the following disclosures: support for attending meetings from Bracco and Bayer; research collaboration with Siemens Healthcare; co-funding by the European Union—FESR or FSE, PON Research and Innovation 2014–2020—DM 1062/2021.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cannella, R., Pilato, G., Mazzola, M. et al. New microvascular ultrasound techniques: abdominal applications. Radiol med 128, 1023–1034 (2023). https://doi.org/10.1007/s11547-023-01679-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01679-6