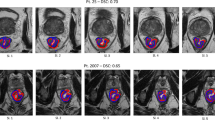
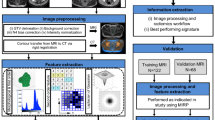
Abstract
Purpose
To explore the variation of the discriminative power of CT (Computed Tomography) radiomic features (RF) against image discretization/interpolation in predicting early distant relapses (EDR) after upfront surgery.
Materials and methods
Data of 144 patients with pre-surgical high contrast CT were processed consistently with IBSI (Image Biomarker Standardization Initiative) guidelines. Image interpolation/discretization parameters were intentionally changed, including cubic voxel size (0.21–27 mm3) and binning (32–128 grey levels) in a 15 parameter’s sets. After excluding RF with poor inter-observer delineation agreement (ICC < 0.80) and not negligible inter-scanner variability, the variation of 80 RF against discretization/interpolation was first quantified. Then, their ability in classifying patients with early distant relapses (EDR, < 10 months, previously assessed at the first quartile value of time-to-relapse) was investigated in terms of AUC (Area Under Curve) variation for those RF significantly associated to EDR.
Results
Despite RF variability against discretization/interpolation parameters was large and only 30/80 RF showed %COV < 20 (%COV = 100*STDEV/MEAN), AUC changes were relatively limited: for 30 RF significantly associated with EDR (AUC values around 0.60–0.70), the mean values of SD of AUC variability and AUC range were 0.02 and 0.05 respectively. AUC ranges were between 0.00 and 0.11, with values ≤ 0.05 in 16/30 RF. These variations were further reduced when excluding the extreme values of 32 and 128 for grey levels (Average AUC range 0.04, with values between 0.00 and 0.08).
Conclusions
The discriminative power of CT RF in the prediction of EDR after upfront surgery for pancreatic cancer is relatively invariant against image interpolation/discretization within a large range of voxel sizes and binning.




Similar content being viewed by others
References
NCCN (2019) NCCN clinical practice guidelines in oncology. Pancreatic adenocarcinoma; Version 1. J Nat Compr Cancer Netw JNCCN 5(10):998–1033. https://doi.org/10.6004/jnccn.2007.0085
Petrelli F, Inno A, Barni S et al (2017) Borderline resectable pancreatic cancer: more than an anatomical concept. Dig Liver Dis 49(2):223–226. https://doi.org/10.1016/j.dld.2016.11.010
Matsumoto I, Murakami Y, Shinzeki M et al (2015) Proposed preoperative risk factors for early recurrence in patients with resectable pancreatic ductal adenocarcinoma after surgical resection: a multi-center retrospective study. Pancreatology 15(6):674–680. https://doi.org/10.1016/j.pan.2015.09.008
Lee Y, Lee J, Yang S et al (2019) Neoadjuvant therapy versus upfront surgery in resectable pancreatic cancer according to intention-to-treat and per-protocol analysis: a systematic review and meta-analysis. Sci Rep 9:15662. https://doi.org/10.1038/s41598-019-52167-9
Barugola G, Partelli S, Marcucci S et al (2009) Resectable pancreatic cancer: who really benefits from resection? Ann Surg Oncol 16:3316–3322. https://doi.org/10.1245/s10434-009-0670-7
Bergquist JR, Puig CA, Shubert CR et al (2016) Carbohydrate antigen 19–9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg 223:52–65. https://doi.org/10.1016/j.jamcollsurg.2016.02.009
Nakamura T, Asano T, Okamura K et al (2019) A preoperative prognostic scoring system to predict prognosis for resectable pancreatic cancer: who will benefit from upfront surgery? J Gastrointest Surg 23(5):990–996. https://doi.org/10.1007/s11605-018-3972-x
Groot V, Rezaee N, Wu W et al (2018) Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 267(5):936–945. https://doi.org/10.1097/SLA.0000000000002234
Barugola G, Frulloni L, Salvia R et al (2009) Is CA 19–9 a screening marker? Dig Liver Dis 41:325–327. https://doi.org/10.1016/j.dld.2009.02.009
Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 3:105–119. https://doi.org/10.3978/j.issn.2078-6891.2011.021
Crippa S, Pergolini I, Javed A et al (2020) Implications of perineural invasion on disease recurrence and survival after pancreatectomy for pancreatic head ductal adenocarcinoma. Ann Surg 276(2):378–385. https://doi.org/10.1097/SLA.0000000000004464
Karmazanovsky G, Gruzdev I, Tikhonova V et al (2021) Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol Med 126(11):1388–1395. https://doi.org/10.1007/s11547-021-01405-0
Marti-Bonmati L, Cerdá-Alberich L, Pérez-Girbés A et al (2022) Pancreatic cancer, radiomics and artificial intelligence. Br J Radiol 95(1137):20220072. https://doi.org/10.1259/bjr.20220072
Palumbo D, Mori M, Prato F et al (2021) Prediction of early distant recurrence in upfront resectable pancreatic adenocarcinoma: a multidisciplinary. Mach Learn-Based Approach Cancers 13(19):4938. https://doi.org/10.3390/cancers13194938
Mori M, Passoni P, Incerti E et al (2020) Training and validation of a robust PET radiomic-based index to predict distant-relapse-free-survival after radio chemotherapy for locally advanced pancreatic cancer. Radiother Oncol 153:258–264. https://doi.org/10.1016/j.radonc.2020.07.003
Chen X, Oshima K, Schott D et al (2017) Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: an exploratory study. PLoS ONE. https://doi.org/10.1371/journal.pone.0178961
Desseroit M, Tixier F, Weber WA et al (2017) 18F- Reliability of PET/CT shape and heterogeneity features in functional and morphological components of non-small cell lung cancer tumors: a repeatability analysis in a prospective multi-center cohort. J Nucl Med 58:406–411. https://doi.org/10.2967/jnumed.116.180919
Zhao B (2021) Understanding sources of variation to improve the reproducibility of radiomics. Front Oncol 11:633176. https://doi.org/10.3389/fonc.2021.633176
Mori M, Cattaneo GM, Muffatti F et al (2019) CT radiomic features of pancreatic neuroendocrine neoplasms (panNEN) are robust against delineation uncertainty. Physica Med 57:41–46. https://doi.org/10.1016/j.ejmp.2018.12.005
Van Velden FH, Kramer GM, Frings V et al (2016) Repeatability of radiomic features in non-small-cell lung cancer [18F]FDG-PET/ CT studies: impact of reconstruction and delineation. Mol Imaging Biol 18(5):788–795. https://doi.org/10.1007/s11307-016-0940-2
Lu L, Ehmke RC, Schwartz LH et al (2016) Assessing agreement between radiomic features computed for multiple CT imaging settings. PLOS ONE 11(12):e0166550. https://doi.org/10.1371/journal.pone.0166550
Linsalata S, Borgheresi R, Marfisi D et al (2022) Radiomics of patients with locally advanced rectal cancer: effect of preprocessing on features estimation from computed tomography imaging. Biomed Res Int 2022:2003286. https://doi.org/10.1155/2022/2003286
Hosseini S, Shiri I, Hajianfar G et al (2021) The impact of preprocessing on the PET-CT radiomics features in non-small cell lung cancer. Front Biomed Technol 8(4):261–272. https://doi.org/10.18502/fbt.v8i4.7754
Hu P, Wang J, Zhong H et al (2016) Reproducibility with repeat CT in radiomics study for rectal cancer. Oncotarget 7(44):71440–71446. https://doi.org/10.18632/oncotarget.12199
Oliver JA, Budzevich M, Zhang GG et al (2015) Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Trans Oncol 8(6):524–534. https://doi.org/10.1016/j.tranon.2015.11.013
Sedlaczek O, Kleesiek J, Gallagher F et al (2022) Quantification and reduction of cross-vendor variation in multicenter DWI MR imaging: results of the cancer core Europe imaging task force. Eur Radiol 32:8617–8628. https://doi.org/10.1007/s00330-022-08880-7
Ibrahim A, Refaee T, Primakov S et al (2021) The effects of in-plane spatial resolution on ct-based radiomic features’ stability with and without combat harmonization. Cancers 13(8):1848. https://doi.org/10.3390/cancers13081848
He L, Huang Y, Ma Z et al (2016) Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Nat Sci Rep 6:34921. https://doi.org/10.1038/srep34921
Larue RTHM, Van De Voorde L, Van Timmeren JE et al (2017) 4DCT imaging to assess radiomics feature stability: an investigation for thoracic cancers. Radiother Oncol 125(1):147–153. https://doi.org/10.1016/j.radonc.2017.07.023
Loi S, Mori M, Benedetti G et al (2020) Robustness of CT radiomic features against image discretization and interpolation in characterizing pancreatic neuroendocrine neoplasms. Physica Med 76:125–133. https://doi.org/10.1016/j.ejmp.2020.06.025
Zwanenburg A, Vallieres M, Abdalah M et al (2020) The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput imagebased phenotyping. Radiology 95(2):328–338. https://doi.org/10.1148/radiol.2020191145
Scalco E, Mori M, Palumbo D, et al. (2022) Limited impact of scanner variability on CT radiomic features in predicting outcome for pancreatic cancer patients. ECR meeting, Vienna
Mim Software Inc. (MIM Software Inc. Cleveland OH). https://www.mimsoftware.com/solutions/radiation-oncology
Tixier F, Le Rest CC, Hatt M et al (2011) Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J. Nucl. Med. 52(3):369–78. https://doi.org/10.2967/jnumed.110.082404
Whybra P, Parkinson C, Foley K et al (2019) Assessing radiomic feature robustness to interpolation in 18F-FDG PET imaging. Sci. Rep. 9(1):9649
Deasy JO, Blanco AI, Clark VH (2003) CERR: a computational environment for radiotherapy research. Med Phys 30(5):979–85. https://doi.org/10.1118/1.1568978
Traverso A, Wee L, Dekker A et al (2018) Repeatability and reproducibility of radiomic features: a systematic review. Int J Radiat Oncol Biol Phys 102(4):1143–1158. https://doi.org/10.1016/j.ijrobp.2018.05.053
Qiu Q, Duan J, Duan Z et al (2019) Reproducibility and non-redundancy of radiomic features extracted from arterial phase CT scans in hepatocellular carcinoma patients: impact of tumor segmentation variability. Quant Imaging Med Surg 9(3):453–464. https://doi.org/10.21037/qims.2019.03.02
Lv W, Yuan Q, Wang Q et al (2018) Robustness versus disease differentiation when varying parameter settings in radiomics features: application to nasopharyngeal PET/CT. Eur Radiol 28(8):3245–3254. https://doi.org/10.1007/s00330-018-5343-0
Benedetti G, Mori M, Panzeri MM et al (2021) CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol Med 126:745–760. https://doi.org/10.1007/s11547-021-01333-z
Acknowledgements
Martina Mori was supported by AIRC (Italian Association for Cancer Research, IG23150).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sara Loi, Diego Palumbo, Martina Mori, Gabriele Palazzo, Claudio Fiorino and Stefano Crippa. The first draft of the manuscript was written by Sara Loi and Claudio Fiorino and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The content of this study was approved by ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. A specific informed consent was signed by the patients whose data are analyzed in the study.
Consent to participate
A specific informed consent was signed by the patients whose data are analyzed in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Loi, S., Mori, M., Palumbo, D. et al. Limited impact of discretization/interpolation parameters on the predictive power of CT radiomic features in a surgical cohort of pancreatic cancer patients. Radiol med 128, 799–807 (2023). https://doi.org/10.1007/s11547-023-01649-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-023-01649-y