Abstract
Purpose
To assess efficacy and safety of cone beam computed tomography (CBCT) in the radiofrequency ablation (RFA) of osteoid osteoma (OO) in children and adolescents, and to compare technical success, clinical success, radiation dose and procedure duration time of CBCT guidance to conventional computed tomography (CT) guidance.
Materials and methods
Between 2015 and 2019, 53 consecutive percutaneous RFA were performed on pediatric patients with CBCT or conventional CT guidance, respectively, in 24 and 29 children and adolescents with 24-month follow-up. Dose area product (DAP) and dose length product (DLP) were recorded, respectively, for CBCT and conventional CT and converted to effective doses (ED).
Results
CBCT and conventional CT groups were similar in terms of patient age and weight, tumor size and tumor location. Technical success was achieved in all cases. Primary clinical success was 91.67% (22/24) for the CBCT group and 89.66% (26/29) for the conventional CT group. Mean DAP was 64.75Gycm2 (range 6.0–266.7). Mean DLP was 972.62mGycm (range 337–2344). ED was significantly lower in the CBCT group compared to the conventional CT group (0.34 mSv vs. 5.53 mSv, p = 0.0119). Procedure duration time was not significantly longer in the CBCT group (102.25 min vs. 92.34 min, p = 0.065). No major complication was registered. Minor complications were observed in 4 patients (2 in CBCT; 2 in conventional CT).
Conclusions
Compared to conventional CT guidance, CBCT guidance for percutaneous OO ablation shows similar technical and clinical success rates, with reduced radiation dose and equivalent procedure duration time. This technique helps sparing dose exposure to pediatric patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoid osteoma (OO) is a benign bone tumor that commonly arises in children and adolescents and is slightly predominant in male population [1]. Generally, it is seen as a little central nidus surrounded by peripheral reactive zone of osteoblasts and thickened cortical bone and fibrotic tissue with vascular elements [2]. The radiographic appearance is typical and consists of a small central radiolucent nidus with bony sclerosis all around, with cortical thickening caused by subperiosteal bone formation [3]. Nowadays, Computed Tomography (CT) is the gold-standard to reach the diagnosis, showing the precise location and size of the nidus. Imaging-guided percutaneous thermal ablation techniques, such as radiofrequency ablation (RFA), cryoablation, microwave ablation (MWA), laser photocoagulation and Magnetic Resonance-guided Focused Ultrasound (MRgFUS), became the first line treatment [4,5,6,7,8]. In particular, CT-guided RFA is the most common technique among percutaneous treatment of OO at present. Accurate placement of the ablation needle is crucial for a complete nidus ablation, even if hard-to-access locations or to low patient compliance make it very challenging and time consuming in certain patients. Limiting exposure of patients to ionizing radiation is necessary in all patients and mandatory in children and adolescents, thus forcing radiologists to lower radiation exposure in case of OO ablation.
Cone beam computed tomography (CBCT) is a new technique that has increasingly been used for imaging-guidance during interventional procedures, including percutaneous bone and muscle tumor ablation [9,10,11,12,13]. Indeed, software allows the CBCT volumetric data to be superimposed on live fluoroscopy, drawing a trajectory for the procedure to be realized under real-time fluoroscopy planning and monitoring [14].
Aim of this study is to assess efficacy and safety of CBCT in the thermal ablation of OO in children and adolescents, and to compare technical success, clinical success, radiation dose and procedure duration time of RFA with CBCT guidance to RFA with conventional CT guidance.
Material and methods
Patients population
Between March 2014 and March 2019, 55 consecutive pediatric patients were evaluated in our Interventional Radiology Department for chronic bone pain with nocturnal exacerbation relieved by salicylates and eventually diagnosed with OO based on radiographic, CT and/or magnetic resonance imaging (MRI) findings and clinical history. Two of them were not eligible to percutaneous thermal ablation because of platelet count < 50,000/µL (1) and coagulation disorder (1). The remaining 53 underwent RFA using CBCT with fluoroscopic guidance overlay (24) or using conventional CT guidance (29). The choice of CBCT versus conventional CT was due to the fact that the CBCT technology was available in our department from the end of 2017, so that the vast majority of CBCT procedures performed in 2018 and 2019, and the majority of conventional CT cases were performed between 2014 and 2017.
Cone beam CT-guided procedure
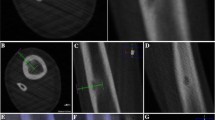
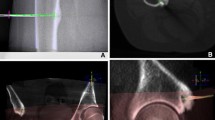
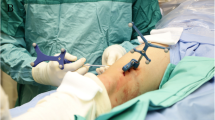
No patient was treated under general anesthesia. Peripheral nerve anesthesia was conducted under ultrasound guidance in patients with OO in the extremities. Local anesthesia (2–5 ml of mepivacaine hydrochloride at 2%) was performed in the cutaneous site of needle penetration. In some cases, subdural anesthesia was performed. An unenhanced CBCT of the anatomic region was performed, and images were reviewed on an adjacent workstation in order to identify target site and skin entrance area on multiplanar reconstructions (MPR). Probe placement trajectory was determined using a navigation system software (SIRIO, Masmec manufactured). The patients are generally placed around one meter under the navigation system arm. A square plastic device with four little spheres in the corners is applied on the insertion body region, and a CT scan is performed so that the insertion point is acquired by the navigation system. Then, a similar plastic device with four spheres is applied on the needle and recognized by the navigation system, which processes and provides the right trajectory to the lesion and a real-time tracking of the advancing needle. After a stab incision, the biopsy trocar (Bone Biopsy System, Bonopty, Radi Medical Device, Sweden) was advanced under intermittent fluoroscopic guidance just into the bone cortex superficial to the lesion. Drilling through the nidus under live fluoroscopic guidance with overlay on CBCT images was mandatory in cases of dense cortical bone (Bonopty drill set, standard length 122 mm, extended length 160 mm, caliber 17 G12/ 1.7-mm). After reaching the nidus, tissue sample was obtained for histology using a biopsy needle (Bonopty biopsy needle, Radi Medical Device, Sweden) inserted through the trocar. Next, the electrode was inserted through the trocar aiming at the center of the nidus of its active tip under imaging-guidance. A RF bipolar ablation system (Covidien) was used to perform RFA by raising the temperature directly to 90–93 °C for around 6 min.
Conventional CT-guided procedure
No patient was treated under general anesthesia. Conventional CT-guided procedures were performed on a GE Optima 64-slice CT (GE Medical Systems, Milwaukee, WI). After administration of local anesthesia (2–5 ml of mepivacaine hydrochloride at 2%) in the cutaneous site of needle penetration, unenhanced CT with multiplanar reconstruction (MPR) was used to localize the lesion and plan the best approach to avoid vital structures. Then, the position was ascertained by CT using a radiopaque landmark, and the skin was marked. A stab incision was made, and a biopsy trocar (Bone Biopsy System, Bonopty, Radi Medical Device, Sweden) was advanced to cortex. Drilling through the cortex was mandatory in cases of dense cortical bone (Bonopty drill set, standard length 122 mm, extended length 160 mm, caliber 17 G12/ 1.7-mm). Once the probe reached the target, a final CT scan was performed in all cases to document probe position, and a lesion tissue sample was obtained for histology using a biopsy needle (Bonopty biopsy needle, Radi Medical Device, Sweden) inserted through the trocar. Next, the electrode was inserted through the trocar aiming at the center of the nidus of its active tip under imaging-guidance. A RF bipolar ablation system (Covidien) was used to perform RFA by raising the temperature directly to 90–93 °C for around 6 min. The total number of scans and images performed varied on the base of the operator’s needs. The total number of scans to reach the lesion varied on the base of the lesion location.
Definitions
Technical success was defined as the correct placement of the electrode in the lesion, so that no portion of the lesion was more than 5–7 mm away from the exposed tip [15]. Clinical success was considered as the complete disappearance of symptoms after the treatment. Residual symptoms were defined as pain or impaired function or both identical to the presenting complaints that persisted for more than 2 weeks after radiofrequency thermal ablation. Recurrent symptoms were defined as the reappearance of symptoms that followed a symptom-free period after RF thermal ablation [16].
Outcome measures
Technical success was considered as the primary outcome in this study. Clinical success, reintervention rate, complications, radiation dose and procedure duration time were also recorded as secondary outcomes. Visual Analog Scale (VAS) for numeric pain score (0–10) was recorded at presentation and during follow-up. Response to analgesics was also recorded.
Primary clinical success was defined as VAS of 1.5/10 or less after a single ablation. Secondary clinical success was defined as VAS of 1.5/10 or less following repeated ablations. Major complications were defined as injuries requiring further therapy, hospitalization, permanent adverse sequelae or death. Minor complications were defined as injuries requiring no further therapy, with no permanent adverse sequelae.
Radiation dose for each procedure was estimated by collecting dose area product (DAP) and dose length product (DLP) and converting them to effective dose (ED) for comparison. The following published conversion factors were considered: (a) for DAP to ED conversion, established conversion coefficients based on phantom models were used, ranging from 0.0034 to 0.0101 mSv/mGy * cm2 [17], considering patient age and area of body scanned [18, 19]; (b) for DLP to ED conversion, established conversion coefficients (k-factors) based on phantom models were used, ranging from 0.0003 to 0.0271 mSv/mGy * cm [17], considering patient age and area of body scanned. The procedure duration time was defined considering the patients’ arrival time in the interventional radiology room and the exit time from the same room.
Statistical analysis
The statistical analysis was performed with MATLAB statistical toolbox version 2008 (MathWorks, Natick, MA, USA) for Windows at 32 bit. Data were categorized as numbers and percentage for qualitative variables and mean and range for quantitative variables. Groups characteristics were compared through Chi-squared test for qualitative variables (gender, tumor location) and with Student’s t test for quantitative variables (age, tumor size). All tests with p value (p) < 0.05 were considered significant.
Results
Tables 1 and 2 show the demographics of the patient population and the tumor characteristics, respectively.
A total of 53 lesions underwent RFA in 53 patients. Twenty-four tumors were ablated using fluoroscopic CBCT guidance, and 29 were ablated using conventional CT guidance. These groups were similar in terms of patient age and weight, tumor size (p value 0.22), tumor location (p value 0.13) and side of the lesion (p value 0.36). The average age was 16.08 years (range: 9–19 years) for CBCT group and 16.59 years (range: 11–19 years) for conventional CT group. The average weight was 52.63 kg (range: 35–78 kg) for CBCT group and 57.31 kg (range: 39–84 years) for conventional CT group. The average size of the lesions was 9.96 mm (range: 6–21 mm) for CBCT group and 10.24 mm (range: 7–19 mm) for conventional CT group. The mean duration of symptoms before ablation was overall 4.21 months (range: 1–8 months, standard deviation: 1.84).
Outcomes are showed in Table 3.
Technical success of 100% was registered in both groups. Primary clinical success was 91.67% (22/24) for the CBCT group and 89.66% (26/29) for the conventional CT group. Secondary clinical success was 100%. Five cases of clinical failure were observed at one-month follow-up (three in the conventional CT group, two in the CBCT group). All these patients were retreated with RFA using the same guidance of the first procedure. None of them needed surgery. In case of CBCT guidance, the mean fluoroscopy time per procedure was 8.1 min (range 3.1–18.7) with a mean DAP of 64.75 Gy * cm2 (range 6.0–266.7). In case of conventional CT guidance procedures, a mean DLP of 972.62 mGy-cm (range 337–2344) was registered. Estimated effective radiation doses were significantly lower in the CBCT group compared to the conventional CT group (0.34 ± 0.40 vs. 5.53 ± 9.74 mSv, p = 0.0119).
The procedure duration time was not significantly longer in the CBCT group compared with the conventional CT group (102.25 vs. 92.34 min, p = 0.065). Post-ablation average duration of pain medication use was 1.1 days (range: 0–7 days, SD: 1.8 days) for CBCT group and 1.1 days (range: 0–7 days, SD: 1.8 days) for conventional CT, and by 1 week post-procedure, all patients had ceased medical therapy.
The procedure was overall well-tolerated. There was no major complication in the CBCT group nor in the conventional CT one. Thermal skin injury with subsequent resolution was described as a minor complication in one patient treated under CBCT guidance, and one treated under conventional CT guidance. Transient foot numbness was complained by one patient in the conventional CT group. Eventually, slight bone infection was found in one patient in the CBCT group and underwent pharmacological treatment. Overall mean follow-up time was at least 24 months (range: 24–31 months).
Discussion
Some previous reports have described successful non-operative treatment of OO [20,21,22]. Nevertheless, the persistence of symptoms forces many patients to undergo definitive surgical or interventional ablative treatment. At present, percutaneous thermal ablation is considered as the standard of treatment in children and adolescents with OO, and RFA is the largely preferred ablative technique in children [23, 24]. Traditionally, CT guidance has been used to perform OO ablation procedures, sometimes with fluoroscopy. On the other hand, C-arm CBCT is a new imaging method that allows acquisition of cross-sectional imaging through the use of using modern flat panel detector angiographic systems. Probe placement trajectory can be planned and monitored thanks to volumetric images superimposed and co-displayed with fluoroscopic imaging in real-time [25]. Recently, the spreading of this newer technology has increased the use of CBCT in many interventional procedures [9, 12, 26, 27].
Compared to conventional CT, the use of CBCT allows a decreased radiation exposure to patients and operators, as showed by results in our series. This fact was proved through the direct comparison of the radiation dose administered to patients during OO ablation. In order to compare different radiating modalities, the recorded radiation output (DLP for conventional CT and DAP for CBCT) was converted to ED, which is generally used to account for the biological effects of radiation. This parameter represents an average dose to tissues adjusted according to organ-weighting factors proposed by the International Commission on Radiological Protection (ICRP) Publication 60 [28].
In recent years, “Image Gently” and “Step Lightly” campaigns well underlined the great importance of limiting radiation exposure as low as possible in pediatric patients [29, 30]. These campaigns comply with the ICRP Publication 60, which indicates the ED as an effective tool for the direct comparison of radiation dose due to different modalities. The ED values in both groups of our series are quite low, probably due to the fact that the great majority of the tumors were in the limbs, which are less radiosensitive than the trunk. This is due to the presence of many soft organs in thorax, abdomen and pelvis, thus increasing the ED conversion factors for these regions. This was an important result due to the fact that limiting ionizing radiation exposure and application of as low as reasonable achievable (ALARA) principles are crucial, particularly in the young patient population in whom OO frequently occurs. Nevertheless, this parameter should be constantly highlighted in the medical report of the procedure in order to avoid medicolegal litigations based on radiation exposure [31]. So far, the only radiation-free ablation technique described for OO is MRgFUS, a needless method that uses the ablative power of ultrasounds, with similar clinical outcome than RFA according to a recent paper by Arrigoni [8].
In patients undergoing OO ablation using conventional CT guidance, our mean DLP of 972.62 mGy-cm (range 337–2344) is markedly greater than what reported by Perry et al. in 2017 (61.5 mGy-cm) [32], but lower in comparison with the values reported by Cheng et al. in 2014 (up to 1058.8 mGy-cm) [33]. This is certainly due to the different lesion location: the great majority of the tumors in the series by Perry et al. [32] were located in the lower extremities (femur, tibia, or foot), which are composed of less radiosensitive tissues, while our series included lesions in trunk and pelvis. On the other side, the difference in comparison to the series by Cheng et al. [33] is probably due to low-dose protocols adopted by our technicians in case of pediatric patients.
Also in patients undergoing OO ablation using CBCT guidance, our mean DAP of 64.75 Gy * cm2 is greater than the one reported in previous published series including only OO in the limbs [32].
Well established conversion coefficients [17,18,19] based on phantom models were used to transform DAP and DLP in ED, thus allowing a comparison between different modalities.
Our mean conventional CT ED of 5.53 mSv (0.27–34.54) is definitely higher than previous published EDs for OO in the limbs (foot, 0.07 ± 0.05; knee, 16 ± 0.12; hip, 3.09 ± 1.37) [34], and slightly smaller than previous series considering whole body lesion locations.
Our mean CBCT ED of 0.34 mSv (0.019–1.51) is slightly superior to previous published values of 0.01–0.15 mSv [35, 36] for patients with OO located only in the extremities.
In general, our study supports the available literature that CBCT offers radiation dose reduction compared to conventional CT in the interventional ablation treatment of OO, which is of great importance in case of young patients.
With regard to the procedure duration time, a systematic review of the literature on RFA found an average ablation time of 6.8 min for treatment of OO [37]. This is in accordance with the ablation time registered in our series, both for CBCT and CT guidance groups. Differently, the total procedure duration time showed a slightly predominance of CBCT procedure, which was not statistically significant (102.25 vs. 92.34 min, p = 0.065). This parameter was intended as the total time spent between patient arrival in the interventional room and patient transfer to his hospital room after the procedure, thus including pre- and post-procedure preparation time. Although not statistically significant, this difference was likely due to a wrong practice in the use of CBCT software during the first three cases of OO, which caused a little prolongation of the procedure without any consequence for the patient. The correction of the wrong practice made the following CBCT procedures as fast as the conventional CT one. Overall, this result is different from what reported by Perry et al. [32], who found the total room utilization time for CBCT significantly greater than with conventional CT guidance and explained this fact with the learning curve for the use of the XperGuide software. A possible reason of this difference is that all procedures in our series were performed in local anesthesia while all procedures in the series by Perry et al. were performed in general anesthesia, thus reducing the patient preparation time in our study compared to the Perry’s one. Another reason is the fact that all interventions in our series were based on clinical and imaging diagnosis, and all diagnoses were histologically confirmed due to intraprocedural biopsy. This was performed in every patient before RFA, in order to disclose any other bone diseases mimicking OO, as described by some authors [38, 39].
With regard to the follow-up time, previous studies showed that recurrences of OO following thermal ablation mostly occur within the first year after the treatment. However, a recent series documented recurrence of OO up to 1.4 years after initially successful RFA [20, 40]. Therefore, our long-term follow-up time of 24 months is largely adequate to assess the initial treatment response and to catch late recurrences, as well.
Despite some potential limitations (lower contrast resolution, more susceptibility to beam-hardening artifacts), CBCT guidance has the undoubtable advantage of sparing dose exposure to patients, mainly thanks to the guidance software providing a real-time trajectory with fluoroscopic overlay.
The clinical success rate of 100% in this series is comparable to similarly powered pediatric RFA [1]. There were no major complications in this study. Overall, the minor complication rate was 7.55%, slightly under the range of 9–22% detected by other studies describing ablative techniques for treatment of OO [32].
The primary limitations of this study are the retrospective design and the single center nature. An update of this study with more cases has already been projected because a larger sample size would add additional power to our results. Another limitation is the lack of indisputable conversion coefficients to compare the radiation dose for different guidance techniques (CBCT and conventional CT). Eventually, a study standardized for the choice of the guidance technique would be desirable in order to avoid any statistical bias.
Contrary, potential strengths of this paper are: (1) the inclusion of lesions both in extremities and in trunk / pelvis; (2) the study population larger than previous published papers on the same topic.
In conclusion, CBCT guidance in the RFA of OO in pediatric patients is safe, highly effective, technically sound and clinically successful as much as conventional CT, with decreased radiation dose administered to little patients and without significant augmentation of procedure duration time. All this makes CBCT with fluoroscopic overlay guidance advisable in the pediatric population undergoing thermal ablation of OO.
References
Earhart J, Wellman D, Donaldson J et al (2013) Radiofrequency ablation in the treatment of osteoid osteoma: results and complications. Pediatr Radiol 43(7):814–819
O’Connell JX, Nanthakumar SS, Nielsen GP, Rosenberg AE (1998) Osteoid osteoma: the uniquely innervated bone tumor. Mod Pathol 11:175–180
Motamedi D, Learch TJ, Ishimitsu DN et al (2009) Thermal ablation of osteoid osteoma: overview and step-by-step guide1. Radiographics 29:2127–2141
de Palma L, Candelari R, Antico E et al (2013) Treatment of osteoid osteoma with CT-guided percutaneous radiofrequency thermoablation. Orthopedics 36(5):e581–e587
Rehnitz C, Sprengel SD, Lehner B et al (2012) CT-guided radiofrequencyablation of osteoid osteoma and osteoblastoma: clinical success and long-term follow up in 77 patients. Eur J Radiol 81(11):3426–3434
Whitmore MJ, Hawkins CM, Prologo JD et al (2016) Cryoablation of osteoid osteoma in the pediatric and adolescent population. J Vasc Interv Radiol 27(2):232–237
Fiore F, Stoia V, Somma F (2020) Surgical recurrence of solitary fibrous tumor of the pleura treated with microwave (MW) thermoablation: A case report. Thorac Cancer 11(2):443–446. https://doi.org/10.1111/1759-7714.13263 (Epub 2019 Dec 26. PMID: 31876364; PMCID: PMC6997023)
Arrigoni F, Spiliopoulos S, de Cataldo C et al (2021) Propensity score matched study comparing percutaneous computed tomography-guided radiofrequency ablation to magnetic resonance-guided focused ultrasound for the treatment of osteoid osteoma. J Vasc Interv Radiol 32(7):1044–1051. https://doi.org/10.1016/j.jvir.2021.03.528 (Epub 2021 Mar 26 PMID: 33775816)
Tselikas L, Joskin J, Roquet F et al (2015) Percutaneous bone biopsies: comparison between flat-panel cone-beam CT and CT-scanguidance. Cardiovasc Intervent Radiol 38(1):167–176
Bocchino M, Valente T, Somma F, de Rosa I, Bifulco M, Rea G (2014) Detection of skeletal muscle metastases on initial staging of lung cancer: a retrospective case series. Jpn J Radiol 32(3):164–171. https://doi.org/10.1007/s11604-014-0281-5 (Epub 2014 Jan 23 PMID: 24452325)
Busser WM, Hoogeveen YL, Veth RP et al (2011) Percutaneous radiofrequency ablation of osteoid osteomas with use of real-time needle guidance for accurate needle placement: a pilot study. Cardiovasc Intervent Radiol 34(1):180–183
Abi-Jaoudeh N, Venkatesan AM, Van der Sterren W et al (2015) Clinical experience with cone-beam CT navigation for tumor ablation. J Vasc Interv Radiol 26(2):214–219
Somma F, Stoia V, D’Angelo R, Fiore F (2021) Imaging-guided radiofrequency ablation of osteoid osteoma in typical and atypical sites: Long term follow up. PLoS ONE 16(3):e0248589. https://doi.org/10.1371/journal.pone.0248589.PMID:33735214;PMCID:PMC7971862
Orth RC, Wallace MJ, Kuo MD (2008) C-arm cone-beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 19(6):814–820
Rosenthal DI, Hornicek FJ, Wolfe MW, Jennings LC, Gebhardt MC, Mankin HJ (1998) Percutaneous radiofrequency coagulation of osteoid osteoma compared with operative treatment. J Bone Joint Surg Am 80(6):815–821
Pinto CH, Taminiau AH, Vanderschueren GM, Hogendoorn PC, Bloem JL, Obermann WR (2002) Technical considerations in CT-guided radiofrequency thermal ablation of osteoid osteoma: Tricks of the trade. Am J Roentgenol 179:1633–1642
Saltybaeva N, Jafari ME, Hupfer M, Kalender WA (2014) Estimates of effective dose for CT scans of the lower extremities. Radiology 273(1):153–159
Castellano IA, McNeill JG, Thorp NC, Dance DR, Raphael MJ (1995) Assessment of organ radiation doses and associated risk for digital bifemoral arteriography. Br J Radiol 68(809):502–507
McParland BJ (1998) A study of patient radiation doses in interventional radiological procedures. Br J Radiol 71(842):175–185
Jayakumar P, Harish S, Nnadi C, Noordeen H, Saifuddin A (2007) Symptomaticresolution of spinal osteoid osteoma with conservative management: imaging correlation. Skeletal Radiol 36(Suppl 1):S72–S76 (Epub 2006 Sep 12)
Kneisl JS, Simon MA (1992) Medical management compared with operative treatment for osteoid-osteoma. J Bone Joint Surg Am 74(2):179–185
Moberg E (1951) The natural course of osteoid osteoma. J Bone Joint Surg Am 33(A(1)):166–170
Donkol RH, Al-Nammi A, Moghazi K (2008) Efficacy of percutaneous radiofrequency ablation of osteoid osteoma in children. Pediatr Radiol 38:180–185
Brown SD, van Sonnenberg E (2007) Issues in imaging-guided tumor ablation in children versus adults. AJR Am J Roentgenol 189:626–632
Orth RC, Wallace MJ, Kuo MD et al (2008) Technology assessment committee of the society of interventional radiology C-arm cone beam CT: general principles and technical considerations for use in interventional radiology. J Vasc Interv Radiol 19(6):814–820
Somma F, Stoia V, Serra N, D’Angelo R, Gatta G, Fiore F (2019) Yttrium-90 trans-arterial radioembolization in advanced-stage HCC: The impact of portal vein thrombosis on survival. PLoS ONE 14(5):e0216935
Somma F, D’Angelo R, Serra N, Gatta G, Grassi R, Fiore F (2015) Use of Ethanol in the Trans-Arterial Lipiodol Embolization (TAELE) of Intermediated-Stage HCC: Is This Safer than Conventional Trans-Arterial Chemo-Embolization (c-TACE)? PLoS ONE 10(6):e0129573
International Commission on Radiological Protection (ICRP) (2007) The 2007 recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann ICRP. 37(2–4):49–50
National Council on Radiation Protection and Measurements. Ionizing radiation exposure of the population of the United States. National Council on Radiation Protection report no. 160.Bethesda, Md: National Council on Radiation Protection and Measurements, 2009.
Johnson C, Martin-Carreras T, Rabinowitz D (2014) Pediatric interventional radiology and dose-reduction techniques. Semin Ultrasound CT MR 35(4):409–414
Pinto F, Capodieci G, Setola FR et al (2012) Communication of findings of radiologic examinations: medicolegal considerations. Semin Ultrasound CT MR 33(4):376–378. https://doi.org/10.1053/j.sult.2012.01.014 (PMID: 22824126)
Perry BC, Monroe EJ, McKay T, Kanal KM, Shivaram G (2017) Pediatric percutaneous osteoid osteoma ablation: cone-beam CT with fluoroscopic overlay versus conventional CT guidance. Cardiovasc Intervent Radiol 40(10):1593–1599. https://doi.org/10.1007/s00270-017-1685-2 (Epub 2017 May 11 PMID: 28497188)
Cheng EY, Naranje SM, Ritenour ER (2014) Radiation dosimetry of intraoperative cone-beam compared with conventional CT for radiofrequency ablation of osteoid osteoma. J Bone Joint Surg Am 96(9):735–742
Biswas D, Bible JE, Bohan M et al (2009) Radiation exposure from musculoskeletal computerized tomographic scans. J Bone Joint Surg Am 91(8):1882–1889
Koivisto J, Kiljunen T, Wolff J, Kortesniemi M (2013) Assessment of effective radiation dose of an extremity CBCT, MSCT and conventional X ray for knee area using MOSFET dosemeters. Radiat Prot Dosimetry 157(4):515–524
Zbijewski W, De Jean P, Prakash P et al (2011) A dedicated cone-beam CT system for musculoskeletal extremities imaging: design, optimization, and initial performance characterization. Med Phys 38(8):4700–4713
Lanza E, Thouvenin Y, Viala P et al (2014) Osteoid osteoma treated by percutaneous thermal ablation: when do we fail? A systematic review and guidelines for future reporting. Cardiovasc Intervent Radiol 37:1530–1539
Boscainos PJ, Cousins GR, Kulshreshtha R et al (2013) Osteoid osteoma. Orthopedics 36:792–800
Iyer RS, Chapman T, Chew FS (2012) Pediatric bone imaging: diagnostic imaging of osteoid osteoma. AJR Am J Roentgenol 198:1039–1052
Shields DW, Sohrabi S, Crane EO et al (2017) Radiofrequency ablation for osteoid osteoma - Recurrence rates and predictive factors. Surgeon 16:156–162
Funding
No funding was received to support this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
This study has been approved by the Scientific Committee of our National Cancer Institute. All procedure were performed in accordance with the ethical standards laid down in the 1964 Declaration of HELSINKI and its later amendments. Appropriate written informed consent was collected before every procedure. Data were retrospectively collected by a dedicated data manager. All authors reviewed and approved this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fiore, F., Somma, F., D’Angelo, R. et al. Cone beam computed tomography (CBCT) guidance is helpful in reducing dose exposure to pediatric patients undergoing radiofrequency ablation of osteoid osteoma. Radiol med 127, 183–190 (2022). https://doi.org/10.1007/s11547-021-01439-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-021-01439-4