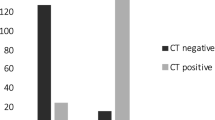Abstract
Objectives
We aimed to assess the diagnostic performance of CT in patients with a negative first RT-PCR testing and to identify typical features of COVID-19 pneumonia that can guide diagnosis in this case.
Methods
Patients suspected of COVID-19 with a negative first RT-PCR testing were retrospectively revalued after undergoing CT.
CT was reviewed by two radiologists and classified as suspected COVID-19 pneumonia, non-COVID-19 pneumonia or negative.
The performance of both first RT-PCR result and CT was evaluated by using sensitivity (SE), specificity (SP), positive predictive value (PPV), negative predictive value (NPV) and area under the curve (AUC) and by using the second RT-PCR test as the reference standard.
CT findings for confirmed COVID-19 positive or negative were compared by using the Pearson chi-squared test (P values < 0.05)
Results
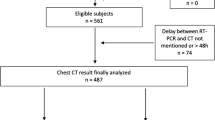
Totally, 337 patients suspected of COVID-19 underwent CT and nasopharyngeal swabs in March 2020. Eighty-seven out of 337 patients had a negative first RT-PCR result; of these, 68 repeated RT-PCR testing and were included in the study.
The first RT-PCR test showed SE 0, SP = 100%, PPV = NaN, NPV = 70%, AUC = 50%, and CT showed SE = 70% SP = 79%, PPV = 86%, NPV = 76%, AUC = 75%.
The most relevant CT variables were ground glass opacity more than 50% and peripheral and/or perihilar distribution.
Discussion
Negative RT-PCR test but positive CT features should be highly suggestive of COVID-19 in a cluster or community transmission scenarios, and the second RT-PCR test should be promptly requested to confirm the final diagnosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On May 24, Italy reported 229,858 confirmed cases of coronavirus disease-19 (COVID-19) and 32,785 deaths since the initial outbreak of the disease in Codogno, Italy, in late February [1].
Clinically, patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) present fever, cough, dyspnea, muscle aches and bilateral pneumonia on imaging [2].
Real-time polymerase chain reaction (RT-PCR) on a nasopharyngeal swab is the most frequently used diagnostic method for detecting SARS-CoV-2 [3], with a sensitivity ranging from 60 to 71% [2, 4, 5]. Due to the relatively low sensitivity, the swab should be repeated on patients who have symptoms and computed tomography (CT) findings suggestive of COVID-19 but a negative test [5, 6].
CT could play a significant role in COVID-19 case screening anticipating RT-PCR positivity [2, 6, 7].
Chest CT could reduce false-negative diagnosis of RT-PCR in the early stages of the disease with a sensitivity of 56–98% in identifying COVID-19 at initial presentation [4,5,6, 8,9,10].
Despite its high sensitivity in diagnosing COVID-19, chest CT had low specificity (25%), as shown in a report of 1014 patients with COVID-19 [2].
Different approaches are possible in managing symptomatic patients following a negative RT-PCR result, as reported in recent guidelines from different countries [11,12,13,14,15,16,17].
According to the Multinational Consensus Statement from the Fleischner Society, in a high pretest probability environment (according to the cluster or community transmission scenarios defined by the World Health Organization [18]) and in case of resource limitations, chest imaging is suggested to provide more rapid identification of patients when RT-PCR COVID-19 testing is not available or initially negative [19].
The rapid identification of patients with COVID-19 is imperative during an outbreak of a highly infectious disease. An initial false-negative result could delay treatment and increase the risk of viral transmission to others.
This study aimed to describe the diagnostic performance of chest CT in patients with a moderate or high pretest probability of COVID-19 infection, with negative RT-PCR testing. We also aimed at identifying imaging features typical of COVID-19 pneumonia diagnosis, which can help suggest a diagnosis in patients with a negative RT-PCR.
Materials and methods
Patient population and study design
The study was approved by our local institutional review board (IRB). Informed consent was waived because of the retrospective nature of the study and the use of anonymous clinical data.
Data were extracted from an institutional prospectively maintained database, including consecutive patients admitted to our Emergency Department, from March 1, 2020, to March 29, 2020.
We included symptomatic patients who underwent CT with a moderate or high pretest probability of COVID-19 infection according to the transmission scenarios (cluster or community) defined by the World Health Organization [19] with moderate or severe respiratory symptoms [11] and a negative RT-PCR swab. The time-interval between chest CT and the RT-PCR assay was no longer than four days.
Clinical data
All patients who underwent CT were symptomatic, presenting with fever (temperature > 37·5 °C), cough, and dyspnea.
A patient was considered as COVID-19 positive or negative after a positive or negative bronchoalveolar lavage or a second nasopharyngeal RT-PCR test.
The preferred choice in our hospital was the RT-PCR test from bronchoalveolar lavage after a first negative RT-PCR swab.
We excluded patients who did not undergo CT examination or without two RT-PCR tests.
Laboratory data
Nasopharyngeal swabs or bronchoalveolar lavage specimens were analyzed with RT-PCR technique to confirm the presence of the SARS-CoV-2 virus in the upper or lower respiratory tract. Two methods were used. One method is based on RNA extraction through high-affinity magnetic silica (Biomérieux, France) and amplification with AllplexTM 2019-nCoV Assay (Seegene, Seoul, South Korea), a multiplex real-time PCR assay for simultaneous detection of 3 target genes of SARS-CoV-2 in a single tube with the CFX96TM real-time PCR instrument (Bio-Rad, France). The assay is designed to detect N, E and RdRP genes. The second system detected the same genes and was performed on the InGenius instrument (GeneFinder COVID-19 Plus RealAmp kit—ELITech Group, South Korea).
CT acquisition technique
As per our hospital COVID-19 protocol, all chest CT acquisitions were obtained with the patients in the supine position during end-inspiration without contrast medium injection. Chest CT was performed on a Philips (Netherlands) Brilliance 64 CT scanner dedicated only to patients with suspected COVID-19. The following technical parameters were used: tube voltage 120 kV; tube current modulation 127 mAs; spiral pitch factor 1·490; rotation time 0·4 s, matrix 512; reconstructions had a slice thickness of 2 mm.
CT image analysis
Two radiologists with five and fifteen years of experience (> 1000 CT per year) in chest imaging, who were blinded to RT-PCR results, reviewed all chest CT images. All patients’ identifying information was removed from the CT studies.
The two radiologists evaluated imaging in consensus in terms of the following parameters [20]: (a) multiple lobe involvement, (b) peripheral or perihilar distribution, (c) upper or lower zone distribution, (d) ground glass opacities (GGO) more than 50% of lung pattern (including crazy paving), (e) consolidation more than 50% of lung pattern, (f) solid nodules, (g) presence of cavitation, (h) ring halo sign, (i) lymphadenopathy (defined as lymph node with short axis > 10 mm), (l) pleural and (m) pericardial effusion.
In consideration of the presence of previous findings, they assigned the perceived likelihood of COVID-19 infection using this classification: suspected COVID-19 pneumonia, non-COVID-19 pneumonia (suggesting other etiology), or negative CT.
If consensus was not reached, a senior radiologist with more than twenty years of chest CT experience made the final decision.
Statistical analysis
Statistical analysis was performed using MATLAB version 2019b (The Mathworks Inc, USA).
The diagnostic performance of both first RT-PCR result and CT was evaluated by using sensitivity (SE), specificity (SP), diagnostic accuracy, positive predictive value (PPV), negative predictive value (NPV) and area under the curve (AUC), considering the second RT-PCR test result as the definite result.
CT findings (and variable values) for patients with positive or negative second RT-PCR results were compared by using nonparametric Kruskal–Wallis test, for continuous variables, and with the Pearson chi-squared test, for categorical variables. P values of < 0·05 were considered statistically significant. Such aggregation was computed by iteratively using the Boruta algorithm [21] and a random forest classifier [22].
The comparison between the medians of the number of days (time) between the onset of symptoms and first naso-oropharyngeal RT-PCR test, chest CT examination, and second RT-PCR test was expressed through a win–tie–loss table, where all the win–tie–losses were validated by the Wilcoxon rank-sign test (95% confidence level).
Results
Patient population and clinical data
Eighty-seven out of 337 patients had a negative first RT-PCR result. Sixty-eight out of 87 patients who repeated RT-PCR testing were included in the study.
Twenty patients out of 68 had a positive second test result, and 48 patients out of 68 had a negative second test result.
Nineteen out of 87 patients who did not repeat the RT-PCR test were excluded.
Patient population data are summarized in Table 1.
CT performance
Of 68 CT examinations, 24 were diagnosed suspected of COVID-19 pneumonia (14 patients had a positive second test and 10 a negative second test), 31 were diagnosed as non-COVID-19 pneumonia (6 patients had a positive second test and 25 a negative second test), and 13 were diagnosed as negative in consideration of the perceived likelihood of COVID-19 (Table 2).
CT findings in suspected COVID-19 pneumonia and non-COVID-19 pneumonia are reported in Tables 3 and 4.
The most discriminative features were: ground glass opacities (GGO) more than 50% of lung pattern (p value = 0·000,040,687), multiple lobe involvement (p value = 0·00,025,824), peripheral (p value = 0·00,035,559), peripheral and perihilar (p value = 0·0,038,061) distribution, bilateral distribution (p value = 0·0,046,384), pleural effusion (p value = 0·010,927), consolidation more than 50% of lung pattern (p value = 0·01,395), lymphadenopathy (defined as lymph node with short axis > 10 mm) (p value = 0·032,051), while the remaining variables did not show any statistical difference between the distribution of COVID-19-positive patients and COVID-19-negative patients. CT findings assessment through statistical testing showed that the most relevant variables are those highlighted by the violet bars in Fig. 1. The classification performance achieved by the most statistically significant variables and the result of aggregating the first three variables (ground glass opacities more than 50% of lung pattern, peripheral and perihilar distribution, peripheral distribution) are shown in Fig. 2 and 3. Note that the addition of more variables (and rules) to the combinations (ground glass opacities more than 50% of lung pattern and peripheral distribution) and (peripheral and perihilar with peripheral distribution) does not increase performance. The time differences between naso-oropharyngeal RT-PCR and CT scan and second RT-PCR testing (BAL or second nasopharyngeal swab) were compared, as shown in Fig. 4.
A 67-year-old man with a negative first RT-PCR result: ground glass opacities more than 50% of the lung pattern with peripheral and bilateral distribution in axial and coronal CT planes (A, B). Images were analyzed with the COPD advanced visualization application in ISP (Philips Medical Systems, Best, The Netherlands). The extent of the aerated lung was in all batches quantified by the percent of lung voxels with attenuation < − 660 Hounsfield units (C)
Sensitivity, specificity, diagnostic accuracy, positive predictive value (PPV), negative predictive value (NPV) and area under the ROC curve (AUC) for statistically significant features. Colored bars allow a visual comparison of the achieved performance (red for performance measures mainly focusing on positive patients, blue for performance measures mainly focusing on negative patients, yellow for performance measures balancing the performance on positive and negative patients)
The first RT-PCR test showed SE 0 (we analyzed patients with a negative first swab), SP 100%, diagnostic accuracy = 70%, PPV = NaN, NPV = 70%, AUC = 50%, CT showed SE = 70%, SP = 79%, diagnostic accuracy = 58%, PPV = 86%, NPV = 76%, AUC = 75% (Table 2).
Twenty out of 68 patients were false-negative cases of the first RT-PCR test, 10 out of 68 patients were false-positive cases of CT, and six out of 68 patients were false-negative cases of CT.
Fifty out of 68 patients were confirmed COVID-19 positive (n.15) or negative (n.35) by RT-PCR from bronchoalveolar lavage (BAL), with a duration from onset of symptoms to BAL of 7·5 ± 0·29 [min = 1, max = 24] days in positive and 7·5 ± 0·25 [min = 2, max = 31] in negative.
Twelve out of 68 patients were confirmed COVID-19 positive (1) or negative (11) only by the second nasopharyngeal RT-PCR swab with the duration from onset of symptoms to second RT-PCR swab of only 5·5 ± 0·78 days.
Six out of 68 patients were confirmed COVID-19 positive (four) or negative (two) by the second oro-nasopharyngeal RT-PCR swab and BAL.
The diagnostic performance of CT in combination with a second RT-PCR test achieved a SE of 100%, SP of 79% PPV of 67%, NPV of 100%, and accuracy of 85% (Table 2).
We repeated the statistical analysis of CT performance without the 12 patients who were confirmed COVID-19 positive or negative only by the second naso-oropharyngeal RT-PCR swab, and we found SE of 68%, SP of 81%, PPV of 83%, NPV of 76% and accuracy of 74% that confirm the better performance of CT than first RT-PCR swab in the identification of false-negative cases.
Of the 20 confirmed positive COVID-19 patients, nine patients had other lung co-infection, and of the 48 confirmed negative COVID-19 patients, 34 had another lung infection (Table 5).
The performance of CT in the differential diagnosis of suspected COVID-19 pneumonia vs. non COVID-19 pneumonia and negative CT showed SE of 70%, SP of 79%, PPV of 59%, NPV of 86% and accuracy of 76% (Table 2).
Discussion
To date, nucleic acid detection with RT-PCR is the “gold standard” for COVID-19 diagnosis despite being associated with a false-negative rate as high as 50% in a single detection [14].
Notwithstanding the high specificity, RT-PCR tests can give false-negative results if the sample contains low viral load due to the technique of sample collection or the time when the sample is collected in the course of the disease.
The use of CT for COVID-19 diagnosis is controversial. It can have a crucial role in the early identification of false-negative patients, orienting medical choice and follow-up in the endemic area.
A meta-analysis of Kim et al. [23] showed that the pooled sensitivity was 94% for CT and 89% for RT-PCR, and the pooled specificity of chest CT was 37%. The authors evaluated the estimated predictive values of CT and RT-PCR for COVID-19 in Italy. The PPV was 24.5% for CT and 95.1% for RT-PCR; the NPV was 96.6% for CT and 97.6% for RT-PCR.
In the studies [2, 24,25,26,27,28] included in the meta-analysis of Kim et al. [23], the diagnostic performance of CT versus RT-PCR was evaluated in patients with positive and negative first RT-PCR, considering CT as a screening tool.
We aimed to focus on patients with a first negative RT-PCR test to evaluate the added value of CT in this condition.
Our results confirmed a higher sensitivity and lower specificity of CT (SE 70%, SP 79%) in respect of a negative RT-PCR test (SE 0%, SP 100%).
The limitations of RT-PCR are false-negative cases, while of CT are false-positive ones. In our experience, the number of false-positive CT cases was lower than the RT-PCR false-negative cases.
The low specificity of CT is due to the overlap of CT imaging features between COVID-19 and other viral pneumonia and lung conditions. In fact, in 3 out of 6 false-negative CT cases, another lung infection was diagnosed.
It should be noted that COVID-19 pneumonia is difficult to distinguish by imaging from influenza A virus, influenza B virus, cytomegalovirus, or other viral pneumonia or bacterial pneumonia and other lung diseases (vasculitis, dermatomyositis and organizing pneumonia) [27,28,29,30].
In a cluster or community transmission scenario, as seen in Northern Italy, when RT-PCR COVID-19 testing is not available or negative, some false-positive cases may be acceptable. A standardized conclusion of a radiological report in terms of suspected COVID-19 pneumonia, pneumonia and negative CT could speed up the diagnostic path of patients, suggesting to clinicians to maintain the patient in isolation, to repeat RT-PCR test or to make a differential diagnosis with other lung infections or other lung diseases.
We evaluated chest CT considering established imaging features that are considered typical of COVID-19 pneumonia [7,8,9,10, 20], classifying lung patterns in suspected COVID-19 pneumonia, non-COVID-19 pneumonia and negative CT [31].
The performance of chest CT in the differential diagnosis of suspected COVID-19 pneumonia vs. non-COVID-19 pneumonia and negative CT was better aggregating the three most statistically significant variables (GGO more than 50%, peripheral and perihilar or peripheral distribution).
The presence of these features at first CT examination could be used as an imaging biomarker to select patients who need a second RT-PCR test (BAL or repeated nasopharyngeal swab every 48–72 h until persistently negative) in cluster or community transmission scenarios even if it would not be beneficial in a low-prevalence region [24]
In consideration of the rapidly spreading epidemic of COVID-19, in cluster or community transmission scenarios, the early identification of any suspicious case is helpful in order to isolate the patients, administer appropriate treatment and control the emergency disease.
Further studies are needed to evaluate the cost-effectiveness of CT, the benefit–risk ratio of radiation exposure in this condition, and to confirm the diagnostic performance of our imaging criteria in the early identification of RT-PCR false-negative patients.
We believe that the negative COVID-19 patients confirmed by BAL or repeated naso-oropharyngeal swab results, in the presence of a radiological pattern suggestive of COVID-19 pneumonia without other lung infection or disease, should be watched and in the future serological tests could help to confirm the final diagnosis.
The limitations of our study were the small cohort of patients, the monocentric experience and the unavailability of lung biopsy specimens to evaluate the relationship between radiological and histopathological findings.
In conclusion, negative RT-PCR test with positive CT features should be highly suggestive of COVID-19 in a cluster or community transmission scenarios, but it would not be beneficial in a low-prevalence region.
CT could speed up the diagnostic path of patients when RT-PCR COVID-19 testing is not available or negative, suggesting to clinicians to maintain the patient in isolation, to repeat RT-PCR test or to make a differential diagnosis with other lung infections or other lung diseases.
Availability of data and material
The authors declare that they had full access to all of the data in this study and the authors take complete responsibility for the integrity of the data and the accuracy of the data analysis.
References
Covid-19 Dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University. Available at: https://coronavirus.jhu.edu/map.html. Accessed May 24, 2020
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. https://doi.org/10.1148/radiol.2020200642
Clinical Specimens: Novel Coronavirus (2019-nCoV) | CDC. Feb 14, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html/ Accessed February 28, 2020
Fang Y, Zhang H, Xie J et al (2020) Sensitivity of chest CT for COVID-19: comparison to RT-PCR. Radiology. https://doi.org/10.1148/radiol.2020200432
Kanne JP, Little BP, Chung JH, Elicker BM, Ketai LH (2020) Essentials for radiologists on COVID-19: An Update-Radiology Scientific Expert Panel. Radiology. https://doi.org/10.1148/radiol.2020200527
Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for Typical 2019-nCoV pneumonia: relationship to Negative RT-PCR testing. Radiology. https://doi.org/10.1148/radiol.2020200343
Shi H, Han X, Jiang N et al (2020) Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 20:425–434
Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. https://doi.org/10.1148/radiol.2020200463
Chung M, Bernheim A, Mei X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295:202–207
Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. https://doi.org/10.1148/radiol.2020200370
National Health Protection Committee. [Novel coronavirus pneumonia diagnosis and treatment plan (Trial Seventh Edition)]. 2020. Available at: https://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed March 25, 2020
Nicastri E, Petrosillo N, Bartoli TA et al (2020) National institute for the infectious diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep 12:8543
WHO guidelines. Technical guidance. March 11, 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/ Accessed April 13, 2020
The interference factors in Coronavirus 2 nucleic acid detection 2020. Available at: https://kns.cnki.net/kcms/detail/50.1167.R.20200317.1710.002.html. Accessed March 25, 2020
King’s Critical Care–Clinical Management of COVID-19. UK summary of evidence. March 9, 2020. Available at: https://www.nwpgmd.nhs.uk/sites/default/files/KCC%2520COVID19%2520Evidence%2520Summary.pdf/ Accessed April 13, 2020
COVID-19: investigation and initial clinical management of possible cases - GOV.UK March 25, 2020. Update April 6. Available at: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection/ Accessed April 13, 2020
Actualizado el documento con las recomendaciones de tratamiento para pacientes con Covid-19. 20 March 2020. Available at: https://www.diariofarma.com/2020/03/20/actualizado-el-documento-con-las-recomendaciones-de-tratamiento-para-pacientes-con-covid-19/ Accessed April 13, 2020
World Health Organization: Critical preparedness, readiness, and response actions for COVID-19. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/critical-preparedness-readiness-and-response-actions-for-covid-, Accessed April 13, 2020
Rubin GD, Haramati LB, Kanne JP et al (2020) The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Radiology. https://doi.org/10.1016/j.chest.2020.04.003
Ng M-Y, Lee EY, Yang J et al (2020) Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiol Cardiothorac Imaging. https://doi.org/10.1148/ryct.2020200034
Kursa MB, Rudnicki WR (2010) Feature selection with the boruta package. J Stat Softw 36(11):1–13
Schonlau M, Zou RY (2020) The random forest algorithm for statistical learning. Stata J 20:3–29
Kim H, Hong H, Yoon SH (2020) Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. https://doi.org/10.1148/radiol.2020201343
Zhu W, Xie K, Lu H, Xu L, Zhou S, Fang S (2020) Initial clinical features of suspected coronavirus disease 2019 in two emergency departments outside of Hubei. J Med Virol, China https://doi.org/10.1002/jmv.25763
Caruso D, Zerunian M, Polici M et al (2020) Chest CT features of COVID-19 in Rome. Radiology, Italy https://doi.org/10.1148/radiol.2020201237
Cheng Z, Lu Y, Cao Q et al (2020) Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai. AJR Am J Roentgenol, China https://doi.org/10.2214/AJR.20.22959
Franquet T (2011) Imaging of pulmonary viral pneumonia. Radiology 260:18–39
Hayden GE, Wrenn KW (2009) Chest radiograph versus computed tomography scan in the evaluation for pneumonia. J Emerg Med 36:266–270
Yoon SH, Lee KH, Kim JY et al (2020) Chest radiographic and CT findings of the 2019 novel coronavirus disease (covid-19): analysis of nine patients treated in Korea. Korean J Radiol 21:494–500
Koo HJ, Lim S, Choe J, Choi S-H, Sung H, Do K-H (2018) Radiographic and CT features of viral pneumonia. Radiographics 38:719–739
Simpson S, Kay UF, Abbara S et al (2020) Radiological society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the american college of radiology, and RSNA. J Thoracic Imaging. https://doi.org/10.1097/RTI.0000000000000524
Acknowledgments
We thank all our colleagues of the Radiology Department. We are also grateful to our radiology technicians and the many frontline medical staff of the Humanitas Research Hospital for their work during this outbreak.
Funding
The authors did not receive any funding for the here presented research.
Author information
Authors and Affiliations
Contributions
C.G., F.M.S., A.R, G.V., E.C., E.C., G.M.F., A.F., M.T.S., A.C., L.B. substantially contributed to the conception or design of the work, and o contributed to the writing and revision of the manuscript, and o approved the final version of the manuscript, and o are accountable for the manuscript’s contents.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no known competing interests associated with this publication.
Ethics approval
This study was approved by our Ethical Committee.
Human and animal rights
Ethical standards This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
Informed consent was waived because of the retrospective nature of the study and the use of anonymous clinical data.
Consent for publication
Informed consent was waived because of the retrospective nature of the study and the use of anonymous clinical data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giannitto, C., Sposta, F.M., Repici, A. et al. Chest CT in patients with a moderate or high pretest probability of COVID-19 and negative swab. Radiol med 125, 1260–1270 (2020). https://doi.org/10.1007/s11547-020-01269-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01269-w