Abstract
Objectives
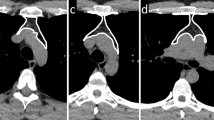
We aimed to assess the ability of radiomics, applied to not-enhanced computed tomography (CT), to differentiate mediastinal masses as thymic neoplasms vs lymphomas.
Methods
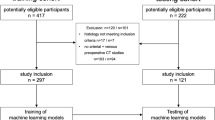
The present study was an observational retrospective trial. Inclusion criteria were pathology-proven thymic neoplasia or lymphoma with mediastinal localization, availability of CT. Exclusion criteria were age < 16 years and mediastinal lymphoma lesion < 4 cm. We selected 108 patients (M:F = 47:61, median age 48 years, range 17–79) and divided them into a training and a validation group. Radiomic features were used as predictors in linear discriminant analysis. We built different radiomic models considering segmentation software and resampling setting. Clinical variables were used as predictors to build a clinical model. Scoring metrics included sensitivity, specificity, accuracy and area under the curve (AUC). Wilcoxon paired test was used to compare the AUCs.
Results
Fifty-five patients were affected by thymic neoplasia and 53 by lymphoma. In the validation analysis, the best radiomics model sensitivity, specificity, accuracy and AUC resulted 76.2 ± 7.0, 77.8 ± 5.5, 76.9 ± 6.0 and 0.84 ± 0.06, respectively. In the validation analysis of the clinical model, the same metrics resulted 95.2 ± 7.0, 88.9 ± 8.9, 92.3 ± 8.5 and 0.98 ± 0.07, respectively. The AUCs of the best radiomic and the clinical model not differed.
Conclusions
We developed and validated a CT-based radiomic model able to differentiate mediastinal masses on non-contrast-enhanced images, as thymic neoplasms or lymphoma. The proposed method was not affected by image postprocessing. Therefore, the present image-derived method has the potential to noninvasively support diagnosis in patients with prevascular mediastinal masses with major impact on management of asymptomatic cases.


Similar content being viewed by others
References
Carter BW, Okumura M, Detterbeck FC, Marom EM (2014) Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol 9:S110–S118. https://doi.org/10.1097/JTO.0000000000000295
Carter BW, Benveniste MF, Madan R et al (2017) ITMIG classification of mediastinal compartments and multidisciplinary approach to mediastinal masses. Radiographics 37:413–436. https://doi.org/10.1148/rg.2017160095
Carter BW, Marom EM, Detterbeck FC (2014) Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 9(9):S102–S109
Dreyling M, Thieblemont C, Gallamini A et al (2013) ESMO consensus conferences: guidelines on malignant lymphoma. Part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol Off J Eur Soc Med Oncol 24:857–877. https://doi.org/10.1093/annonc/mds643
Eichenauer DA, Engert A, Andre M et al (2014) Hodgkin’s lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii70–iii75. https://doi.org/10.1093/annonc/mdu181
Ghielmini M, Vitolo U, Kimby E et al (2013) ESMO guidelines consensus conference on malignant lymphoma 2011 part 1: diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL) and chronic lymphocytic leukemia (CLL). Ann Oncol Off J Eur Soc Med Oncol 24:561–576. https://doi.org/10.1093/annonc/mds517
Kattach H, Hasan S, Clelland C, Pillai R (2005) Seeding of stage I thymoma into the chest wall 12 years after needle biopsy. Ann Thorac Surg 79:323–324. https://doi.org/10.1016/j.athoracsur.2003.08.004
Nagasaka T, Nakashima N, Nunome H (1993) Needle tract implantation of thymoma after transthoracic needle biopsy. J Clin Pathol 46:278–279
Sollini M, Antunovic L, Chiti A, Kirienko M (2019) Towards clinical application of image mining: a systematic review on artificial intelligence and radiomics. Eur J Nucl Med Mol Imaging 46(13):2656–2672. https://doi.org/10.1007/s00259-019-04372-x
Yasaka K, Akai H, Abe O et al (2018) Quantitative computed tomography texture analyses for anterior mediastinal masses: differentiation between solid masses and cysts. Eur J Radiol 100:85–91. https://doi.org/10.1016/j.ejrad.2018.01.017
Yasaka K, Akai H, Nojima M et al (2017) Quantitative computed tomography texture analysis for estimating histological subtypes of thymic epithelial tumors. Eur J Radiol 92:84–92. https://doi.org/10.1016/j.ejrad.2017.04.017
Iannarelli A, Sacconi B, Tomei F et al (2018) Analysis of CT features and quantitative texture analysis in patients with thymic tumors: correlation with grading and staging. Radiol Med 123:345–350. https://doi.org/10.1007/s11547-017-0845-4
Lee HS, Oh JS, Park YS et al (2016) Differentiating the grades of thymic epithelial tumor malignancy using textural features of intratumoral heterogeneity via (18)F-FDG PET/CT. Ann Nucl Med 30:309–319. https://doi.org/10.1007/s12149-016-1062-2
Nakajo M, Jinguji M, Shinaji T et al (2018) Texture analysis of 18F-FDG PET/CT for grading thymic epithelial tumours: usefulness of combining SUV and texture parameters. Br J Radiol 91:20170546. https://doi.org/10.1259/bjr.20170546
Hatt M, Cheze-le Rest C, van Baardwijk A et al (2011) Impact of tumor size and tracer uptake heterogeneity in (18)F-FDG PET and CT non-small cell lung cancer tumor delineation. J Nucl Med 52:1690–1697. https://doi.org/10.2967/jnumed.111.092767
Nioche C, Orlhac F, Boughdad S et al (2018) Lifex: a freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res 78:4786–4789. https://doi.org/10.1158/0008-5472.CAN-18-0125
Sharma A, Paliwal KK (2015) Linear discriminant analysis for the small sample size problem: an overview. Int J Mach Learn Cybern. https://doi.org/10.1007/s13042-013-0226-9
Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Kirienko M, Cozzi L, Antunovic L et al (2018) Prediction of disease-free survival by the PET/CT radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging 45:207–217. https://doi.org/10.1007/s00259-017-3837-7
Bae KT (2010) Intravenous contrast medium administration and scan timing at CT: considerations and approaches. Radiology 256:32–61. https://doi.org/10.1148/radiol.10090908
He L, Huang Y, Ma Z et al (2016) Effects of contrast-enhancement, reconstruction slice thickness and convolution kernel on the diagnostic performance of radiomics signature in solitary pulmonary nodule. Sci Rep 6:34921. https://doi.org/10.1038/srep34921
Cozzi L, Comito T, Fogliata A et al (2019) Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS ONE 14:e0210758. https://doi.org/10.1371/journal.pone.0210758
Cozzi L, Dinapoli N, Fogliata A et al (2017) Radiomics based analysis to predict local control and survival in hepatocellular carcinoma patients treated with volumetric modulated arc therapy. BMC Cancer 17:829. https://doi.org/10.1186/s12885-017-3847-7
Hernández B, Parnell A, Pennington SR (2014) Why have so few proteomic biomarkers “survived” validation? (sample size and independent validation considerations). Proteomics 14:1587–1592. https://doi.org/10.1002/pmic.201300377
Sollini M, Cozzi L, Ninatti G et al (2020) PET/CT radiomics in breast cancer: mind the step. Methods. https://doi.org/10.1016/j.ymeth.2020.01.007
Park JE, Kim D, Kim HS et al (2019) Quality of science and reporting of radiomics in oncologic studies: room for improvement according to radiomics quality score and TRIPOD statement. Eur Radiol. https://doi.org/10.1007/s00330-019-06360-z
Zwanenburg A (2019) Radiomics in nuclear medicine: robustness, reproducibility, standardization, and how to avoid data analysis traps and replication crisis. Eur J Nucl Med Mol Imaging 46(13):2638–2655
Sollini M, Gelardi F, Matassa G et al (2020) Interdisciplinarity: an essential requirement for translation of radiomics research into clinical practice – a systematic review focused on thoracic oncology. Rev Esp Med Nucl Imagen Mol. https://doi.org/10.1016/j.remn.2019.10.003
Sollini M, Cozzi L, Antunovic L et al (2017) PET radiomics in NSCLC: state of the art and a proposal for harmonization of methodology. Sci Rep 7:358. https://doi.org/10.1038/s41598-017-00426-y
Acknowledgements
MK PhD scholarship was funded by the AIRC Grant (IG-2016-18585). The scientific guarantor of this publication is Arturo Chiti, who is the Principal Investigator of this retrospective trial.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Contributions
MK, MS and AC conceptualized the study; GN participated to data collection and image processing; LC performed data analysis; EV, PZ, CCS, FR participated in patient selection and were in charge of treatment; NG and LB participated to patient selection; MK and MS supervised image processing, critically interpreted the results and drafted the paper; IB, LB and AC supervised the activities; and all the authors read, commented and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors of this manuscript declare relationships with the following companies: Kirienko, Ninatti, Voulaz, Gennaro, Barajon, Ricci, Sollini, Balzarini—none. Cozzi—acts as Scientific Advisor to Varian Medical Systems outside the scope of the submitted work. Carlo Stella—received speaker honoraria from MSD, BMS, Amgen, Janssen, AstraZeneca; acted as scientific advisor for Genenta Science, ADC Therapeutics, Sanofi, Boehringer Ingelheim; benefited from an unrestricted Grant from Rhizen Pharmaceuticals. These honoraria and Grants are outside the scope of the submitted work. Zucali—received speaker honoraria from Astellas, Pfizer, Sanofi, Janssen, BMS, Ipsen, and Novartis; acted as scientific advisor for Astellas, Pfizer, BMS, Janssen, MSD and Novartis. All honoraria and Grants are outside the scope of the submitted work. Chiti—received speaker honoraria from General Electric and Blue Earth Diagnostics, acted as scientific advisor for Blue Earth Diagnostics and Advanced Accelerator Applications, and benefited from an unconditional Grant from Sanofi to Humanitas University. All honoraria and Grants are outside the scope of the submitted work. As above mentioned, all declared honoraria and Grants are outside the scope of the submitted work.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Humanitas Clinical and Research Center Review Board approval was obtained, authorization number 3/18, April 17, 2018) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived by the institutional review board.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kirienko, M., Ninatti, G., Cozzi, L. et al. Computed tomography (CT)-derived radiomic features differentiate prevascular mediastinum masses as thymic neoplasms versus lymphomas. Radiol med 125, 951–960 (2020). https://doi.org/10.1007/s11547-020-01188-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-020-01188-w