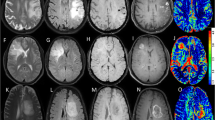
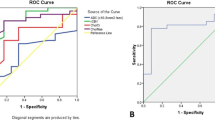
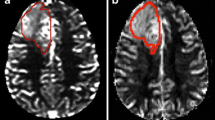
Abstract
Purpose
Evaluation of cerebral blood volume (CBV) with magnetic resonance (MR) imaging can differentiate low-grade from high-grade gliomas. The percentage of signal recovery (PSR) in the venous phase of perfusion curves is inversely proportional to blood–brain barrier (BBB) permeability. Since even BBB permeability relates to glioma malignancy grade, we carried out a comparative evaluation between CBV and PSR to characterise cerebral gliomas.
Materials and methods
Forty-nine patients with cerebral gliomas were studied with MR perfusion imaging. In all tumours, both maximum CBV and minimum PSR were calculated. The difference between the CBV and PSR mean values among the low-grade and high-grade gliomas was assessed using statistical methods. We also examined whether there was an additional difference between low-grade and grade III gliomas. Finally, CBV and PSR diagnostic sensitivity and specificity in identifying low-grade gliomas compared to all gliomas and low-grade gliomas compared to all gliomas excluding glioblastomas was assessed.
Results
A significant difference between low-grade and high-grade gliomas with both CBV and PSR was demonstrated. Conversely, there was a significant difference between low-grade and grade III gliomas only with PSR, while CBV did not show significant difference. Finally, superior sensitivity and specificity of PSR compared to CBV in identifying low-grade gliomas was demonstrated both compared to all gliomas and all gliomas excluding glioblastomas.
Conclusion
The PSR evaluation proved better than CBV for determining the grade of brain and is therefore a useful tool to be considered in the MR evaluation of gliomas.





Similar content being viewed by others
References
Rutten EH, Doesburg WH, Slooff JL (1992) Histologic factors in the grading and prognosis of astrocytoma grade I-IV. J Neurooncol 13:223–230
Law M, Yang S, Babb JS et al (2004) Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 25:746–755
Folkman J (1992) The role of angiogenesis in tumor growth. Semin Cancer Biol 3:65–71
Nugent LJ, Jain RK (1984) Extravascular diffusion in normal and neoplastic tissues. Cancer Res 44:238–244
Shin JH, Lee HK, Kwun BD et al (2002) Using relative blood flow and volume to evaluate histopathologic grade of cerebral gliomas: preliminary results. AJR Am J Roentgenol 179:783–789
Law M, Yang S, Wang H et al (2003) Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 24:1989–1998
Hakyemez B, Erdogan C, Ercan I et al (2005) High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 60:493–502
Cha S, Lupo JM, Chen MH et al (2007) Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 28:1078–1084
Barajas RF, Chang JS, Segal MR et al (2009) Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 253:486–496
Mangia R, Kolar B, Zhu T et al (2011) Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesion of the brain. AJNR Am J Neuroradiol 32:1004–1010
Wetzel SG, Cha S, Johnson G et al (2002) Relative cerebral blood volume measurements in intracranial mass lesions: interobserver and intraobserver reproducibility study. Radiology 224:797–803
Scott JN, Brasher PM, Sevick RJ et al (2002) How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 59:947–949
Cha S (2009) Neuroimaging in neuro-oncology. Neurotherapeutics 6:465–477
Dean BL, Drayer BP, Bird CR et al (1990) Gliomas: classification with MR imaging. Radiology 174:411–415
Watanabe M, Tanaka R, Takeda N (1992) Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology 34:463–469
Aronen HJ, Gazit IE, Louis DN (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Jain R (2011) Perfusion CT imaging of brain tumors: an overview. AJNR Am J Neuroradiol 32:1570–1577
Lefranc M, Monet P, Desenclos C et al (2012) Perfusion MRI as a neurosurgical tool for improved targeting in stereotactic tumor biopsies. Stereotact Funct Neurosurg 90:240–247
Burger P (1986) Malignant astrocytic neoplasms: classification, pathology, anatomy and response to therapy. Semin Oncol 13:16–20
Sugahara T, Korogi Y, Kochi M et al (1998) Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentegenol 171:1479–1486
Knopp EA, Cha S, Johnson G et al (1999) Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 211:791–798
Emblem KE, Nedregaard B, Nome T et al (2008) Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology 247:808–817
Roberts HC, Roberts TP, Brasch RC, Dillon WP (2000) Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: correlation with histologic grade. AJNR Am J Neuroradiol 21:891–899
Jackson A, Kassner A, Annesley-Williams D et al (2002) Abnormalities in the recirculation phase of contrast agent bolus passage in cerebral gliomas: comparison with relative blood volume and tunor grade. AJNR Am J Neuroradiol 23:7–14
Provenzale JM, York G, Moya MG et al (2006) Correlation of relative permeability and relative cerebral blood volume in high-grade cerebral neoplasms. AJR Am J Roentegenol 187:1036–1042
Law M, Young R, Babb J et al (2006) Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modelling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 27:1975–1982
Cha S, Yang L, Johnson G et al (2006) Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR Am J Neuroradiol 27:409–417
Lupo JM, Cha S, Chang SM, Nelson SJ (2005) Dynamic susceptibility-weighted perfusion imaging of high-grade gliomas: characterization of spatial heterogeneity. AJNR Am J Neuroradiol 26:1446–1454
Conflict of interest
The authors declare no conflict of interest.
Ethical standards
The results of this article were derived from a series of human participants all with informed consent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aprile, I., Giovannelli, G., Fiaschini, P. et al. High- and low-grade glioma differentiation: the role of percentage signal recovery evaluation in MR dynamic susceptibility contrast imaging. Radiol med 120, 967–974 (2015). https://doi.org/10.1007/s11547-015-0511-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-015-0511-7