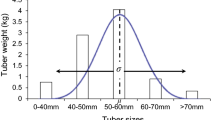
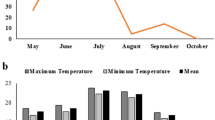
Abstract
The genetic diversity of crop wild relatives is a rich source of valuable genes for plant breeding. Potato wild relatives are an important potential source for breeding programmes focused on heat resistance due to their wider adaptability to different climatic conditions. Wild potato accessions from Embrapa (Brazilian Agricultural Research Corporation) Potato Genebank were assessed and compared to dihaploids and tetraploid cultivated genotypes by measuring tuber yield-related traits and then by analysing through mixed models using the restricted maximum likelihood (REML)/best linear unbiased predicted (BLUP) procedure under heat stress. So, the present study aimed to select the most productive wild potato genotypes under two ranges of temperatures by investigating adaptability and stability of parameters through mixed modelling. Twenty-one genotypes comprising 17 wild potatoes (thirteen diploid Solanum chacoense, one triploid S. chacoense and two diploid S. commersonii), four dihaploid S. tuberosum and one tetraploid commercial cultivar of S. tuberosum (BRSIPR Bel) were evaluated under favourable crop temperature and heat stress conditions using a randomised complete block design. Significant differences were observed for the effects of genotypes and the G × E interaction. Broad sense heritability ranged from 0.24 to 0.59. Genotypic variance was the largest component of phenotypic variance, followed by environmental variance and interaction variance. We observed the highest genotypic correlation by dry matter content. Accuracy of selecting wild genotypes was high for all traits. The genotypes BGB088 (dihaploid S. tuberosum) and BGB113 (diploid S. chacoense) performed as the best ones in most of the studied traits under heat stress. These genotypes show better stability (HMGV), adaptability (RPGV and RPGV*GM), and stability and adaptability of genetic values (HMRPGV and HMRPGV*GM) under high temperature by mixed model methodologies. Conversely, BGB009 and BGB045 (diploid S. commersonii) and BGB086 (triploid S. chacoense) showed consistence in ranking among the ones last for genotypic values for all methodologies. Thus, we concluded that BGB088 and BGB113 are promising genotypes of interest to further studies for providing higher tuber yield under heat stress or non-favourable potato crop environmental conditions. These genotypes should be further assessed in efforts to evaluate the crossability and proceed introgression essays towards broadening the genetic basis of potato crop available for breeding to achieve more resilient cultivars under abiotic stress conditions.









Similar content being viewed by others
References
Aien A, Bahuguna RN, Khetarpal S, Pal Singh M (2011) Higher glycinebetaine and antioxidant enzymes activity are associated with high temperature tolerance in potato. Indian J Plant Physiol 16:285–293
Ali A, Jansky S (2015) Fine screening for resistance to cold-induced sweetening in potato hybrids containing Solanum raphanifolium germplasm. Adv Agric 2015. https://doi.org/10.1155/2015/327969
Annicchiarico P (1992) Cultivar adaptation and recommendation from alfalfa trials in Northern Italy. J Genet Breed 46:269
Bailey-Serres J, Parker JE, Ainsworth EA et al (2019) Genetic strategies for improving crop yields. Nature 575:109–118
Bamberg JB, Del Rio A (2005) Conservation of genetic resources. In: 2005. Genetic improvement of solanaceous crops vol. 1: potato. Science, p 451
Barbosa MHP, Ferreira A, de Resende MDV et al (2014) Selection of sugar cane families by using BLUP and multi-diverse analyses for planting in the Brazilian savannah. Gene Mol Res 13(1):1619–1626. https://doi.org/10.4238/2014.March.12.14
Bashir I, Nicolao R, Heiden G (2021) Wild potatoes: a genetic reservoir for potato breeding. In: Wild germplasm for genetic improvement in crop plants. Elsevier, pp 215–240
Bernardo R (1996) Best linear unbiased prediction of maize single-cross performance. Crop Sci 36:50–56
Bernardo R (1996) Best linear unbiased prediction of maize single-cross performance. Crop Sci 36:50–56
Bethke PC, Halterman DA, Jansky S (2017) Are we getting better at using wild potato species in light of new tools? Crop Sci 57:1241–1258
Bilski JJ, Nelson DC, Conlon RL (1988) Response of six wild potato species to chloride and sulfate salinity. Am Potato J 65:605
Bisognin DA, Müller DR, Streck NA et al (2008) Desenvolvimento e rendimento de clones de batata na primavera e no outono. Pesqui Agropecuária Bras 43:699–705
Bonierbale M (2007) Procedures for standard evaluation trials of advanced potato clones. An international cooperator’s guide. International Potato Center
Borges V, Ferreira PV, Soares L et al (2010) Seleção de clones de batata-doce pelo procedimento REML/BLUP. Acta Sci Agron 32:643–649
Carvalho LCB, Damasceno-Silva KJ, de Rocha M, M, Oliveira GCX, (2017) Genotype x environment interaction in cowpea by mixed models1. Rev Ciência Agronômica 48:872–878
Castro CM, da Pereira A, S, Costa DM, et al (2006) Wild potato genetic resources conserved in Southern Brazil: current knowledge and future perspectives. VI International Solanaceae Conference: Genomics Meets Biodiversity 745:323–330
Ceballos H, Pérez JC, Joaqui Barandica O et al (2016) Cassava breeding I: the value of breeding value. Front Plant Sci 7:1227
da Costa LC, Bisognin DA, Andriolo JL et al (2007a) Identificação de clones de batata com potencial para mesa e adaptados para os cultivos de outono e primavera do Rio Grande do Sul. Ciência e Nat 29:93–104
da Costa LC, Bisognin DA, Andriolo JL, Ritter CEL, Bandinelli MG et al (2007b) Identificação de clones de batata com potencial para mesa e adaptados para os cultivos de outono e primavera do Rio Grande do Sul. Pesqui Agropecuária Bras 48:3324. https://doi.org/10.5902/2179460X9833
da Silva GO, Castro CM, Terres LR et al (2012) Desempenho agronômico de clones elite de batata. Hortic Bras 30:557–560
da Silva GO, de Souza VQ, da Silva Pereira A et al (2006) Early generation selection for tuber appearance affects potato yield components. Crop Breed Appl Biotechnol 6:73–78
de Carvalho ADF, Neto RF, Geraldi IO (2008) Estimation and prediction of parameters and breeding values in soybean using REML/BLUP and least squares. Crop Breed Appl Biotechnol 8:219–224
de Pelegrin AJ, Carvalho IR, Nunes ACP et al (2017) Adaptability, stability and multivariate selection by mixed models. Am J Plant Sci 8:3324
de Resende MDV (2002) Genética biométrica e estatística no melhoramento de plantas perenes. Embrapa Informação Tecnológica, Colombo: Embrapa Florestas
de Resende MDV (2004) Métodos estatísticos ótimos na análise de experimentos de campo. Embrapa Florestas-Documentos (INFOTECA-E)
Deva Rodrigues (2017) Alelo Portal strengthens national and international institutions’ databases. Plant Prod
Douches DS, Maas D, Jastrzebski K, Chase RW (1996) Assessment of potato breeding progress in the USA over the last century. Crop Sci 36:1544–1552
Dutt S, Manjul AS, Raigond P et al (2017) Key players associated with tuberization in potato: potential candidates for genetic engineering. Crit Rev Biotechnol 37:942–957
Fahad S, Bajwa AA, Nazir U et al (2017) Crop production under drought and heat stress: plant responses and management options. Front Plant Sci 8:1147
Farias Neto JT de, Moura EF, de Resende MDV et al (2013) Genetic parameters and simultaneous selection for root yield, adaptability and stability of cassava genotypes. Pesqui agropecuária Bras 48:1562–1568. https://doi.org/10.1590/S0100-204X2013001200005
Fumia N, Pironon S, Rubinoff D et al (2022) Wild relatives of potato may bolster its adaptation to new niches under future climate scenarios. Food Energy Secur e360. https://doi.org/10.1002/fes3.360
George TS, Taylor MA, Dodd IC, White PJ (2017) Climate change and consequences for potato production: a review of tolerance to emerging abiotic stress. Potato Res 60:239–268
Gonçalves GM, Viana AP, Amaral Junior AT do, Resende MDV de (2014) Breeding new sugarcane clones by mixed models under genotype by environmental interaction. Sci Agric 71:66–71 https://doi.org/10.1590/S0103-90162014000100009
Hancock RD, Morris WL, Ducreux LJM et al (2014) Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell Environ 37:439–450
Hanneman RE Jr (1996) Evaluation of wild species for new sources germplasm that chip directly from cold storage. Am J Potato Res 73:360
Haverkort AJ, Struik PC (2015) Yield levels of potato crops: recent achievements and future prospects. Field Crops Res 182:76–85. https://doi.org/10.1016/j.fcr.2015.06.002
Haynes KG, Haynes FL, Henderson WR (1989) Heritability of specific gravity of diploid potato under high temperature growing conditions. Crop Sci 29:622–625
IPCC (2014) Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp
Karimi V, Karami E, Keshavarz M (2018) Climate change and agriculture: impacts and adaptive responses in Iran. J Integr Agric 17:1–15
Lawson C, Burton H, Humphries F (2018) The important place of information in the evolving legal and policy framework for the conservation and sustainable use of the world’s plant genetic resources for food and agriculture
Lehretz GG, Sonnewald S, Hornyik C et al (2019) Post-transcriptional regulation of FLOWERING LOCUS T modulates heat-dependent source-sink development in potato. Curr Biol 29:1614–1624
Li PH (1977) Frost killing temperatures of 60 tuber-bearing Solanum species. American Potato Journal 54:452–456
Lin C-S, Binns MR (1988) A superiority measure of cultivar performance for cultivar × location data. Can J Plant Sci 68:193–198
Lizana XC, Avila A, Tolaba A, Martinez JP (2017) Field responses of potato to increased temperature during tuber bulking: projection for climate change scenarios, at high-yield environments of Southern Chile. Agric for Meteorol 239:192–201
Machida-Hirano R (2015) Diversity of potato genetic resources. Breed Sci 65:26–40. https://doi.org/10.1270/jsbbs.65.26
Mendes FF, Guimarães LJM, Souza JC et al (2012) Adaptability and stability of maize varieties using mixed model methodology. Crop Breed Appl Biotechnol 12:111–117
Muthoni J, Kabira JN (2015) Potato production in the hot tropical areas of Africa: progress made in breeding for heat tolerance. Int J Biol 7:220
Oliveira R de S, Ribeiro CVG, Neres DF, et al (2020) Evaluation of genetic parameters and clonal selection of Eucalyptus in the Cerrado region. Crop Breed Appl Biotechnol 20:
Olivoto T, Nardino M, Carvalho IR et al (2017) REML/BLUP and sequential path analysis in estimating genotypic values and interrelationships among simple maize grain yield-related traits. Genet Mol Res 16:1–19
Pacheco JE, Urquijo JS, Darghan AE, Rodríguez LE (2020) BLUP (Best Linear Unbiased Predictors) analysis for the selection of superior genotypes in the yellow diploid potato (Solanum tuberosum group Phureja). Rev Colomb Ciencias Hortícolas 14:
Paul S, Farooq M, Bhattacharya SS, Gogoi N (2017) Management strategies for sustainable yield of potato crop under high temperature. Arch Agron Soil Sci 63:276–287
Pereira AS, Nazareno NRX, Silva GO et al (2015) “BRSIPR Bel”: a chip-processing potato cultivar with tubers of good appearance. Hortic Bras 33:135–139
Piepho H-P (1994) Best linear unbiased prediction (BLUP) for regional yield trials: a comparison to additive main effects and multiplicative interaction (AMMI) analysis. Theor Appl Genet 89:647–654
Piepho HP, Möhring J, Melchinger AE, Büchse A (2008) BLUP for phenotypic selection in plant breeding and variety testing. Euphytica 161:209–228
Pino MT, Ávila A, Molina A, et al (2013) Enhanced in vitro drought tolerance of Solanum tuberosum and Solanum commersonii plants overexpressing the ScCBF1 gene. Int J Agric Nat Resour 40:171–184
Pupin S, dos Santos A, Gonzalez Zaruma DU et al (2015) Productivity, stability and adaptability in open pollination progenies of Eucalyptus urophylla ST Blake. Sci For 127–134
Purba AR, Flori A, Baudouin L, Hamon S (2001) Prediction of oil palm (Elaeis guineensis, Jacq.) agronomic performances using the best linear unbiased predictor (BLUP). Theor Appl Genet 102:787–792
Quiroz R, Ramírez DA, Kroschel J et al (2018) Impact of climate change on the potato crop and biodiversity in its center of origin. Open Agric 3:273–283
Raymundo R, Asseng S, Prassad R et al (2017) Performance of the SUBSTOR-potato model across contrasting growing conditions. Field Crops Res 202:57–76
Raymundo R, Asseng S, Robertson R et al (2018) Climate change impact on global potato production. Eur J Agron 100:87–98
de Resende MDV, Duarte JB (2007) Precision and quality control cultivars evaluation experiments. Trop Agric Res 37:182–194
Ross H, Hunnius W (1986) Potato breeding: problems and perspectives. Fortschritte der Pflanzenzüchtung 13:132 p.issn 0301-2727
Sabbah S, Tal M (1995) Salt tolerance in Solanum kurzianum and S. tuberosum cvs Alpha and Russet Burbank. Potato Res 38:319–330
Seid E, Mohammed W, Abebe T (2020) Genetic variability, heritability and genetic advance in potato (Solanum tuberosum L.) for processing quality, yield and yield related traits. Int J Plant Breed Crop Sci 7:928–936
Silva GO, Pereira AS, Azevedo FQ, Carvalho ADF (2018) Genotypic selection of advanced potato clones by REML/BLUP. Hortic Bras 36:265–270
Singh A, Kaushal N, Sharma R et al (2016) Effect of elevated temperature on in vitro microtuberization of potato genotypes with different thermotolerance levels. Vegetos 29:3. https://doi.org/10.5958/2229-4473.2016
Singh A, Siddappa S, Bhardwaj V et al (2015) Expression profiling of potato cultivars with contrasting tuberization at elevated temperature using microarray analysis. Plant Physiol Biochem 97:108–116
Sociedade Brasileira de Ciência do Solo (2004) Manual de adubação e de calagem para os RS/SC, 10th edn. Comissão de Química e Fertilidade do Solo, Porto Alegre
Sousa T de JF de, Rocha M de M, Damasceno-Silva KJ et al (2019) Simultaneous selection for yield, adaptability, and genotypic stability in immature cowpea using REML/BLUP. Pesqui Agropecuária Bras 54. https://doi.org/10.1590/S1678-3921.pab2019.v54.01234
Souza YP de, Santos PR dos, Nascimento MR et al (2018) Assessing the genotypic performance of carioca beans through mixed models. Ciência Rural 48(7). https://doi.org/10.1590/0103-8478cr20170761
Steiner JL, Briske DD, Brown DP, Rottler CM (2018) Vulnerability of Southern Plains agriculture to climate change. Clim Change 146:201–218
Sturion JA, de Resende MDV (2005) Seleção de progênies de erva-mate (Ilex paraguariensis St. Hill.) para a produtividade, estabilidade e adaptabilidade temporal de massa foliar. Bol. pesq Fl. Colombo 50:37–51
Tang R, Niu S, Zhang G et al (2018) Physiological and growth responses of potato cultivars to heat stress. Botany 96:897–912
Ticona-Benavente CA, da Silva Filho DF (2015) Comparison of BLUE and BLUP/REML in the selection of clones and families of potato (Solanum tuberosum). 14(4):18421–18430. http://dx.doi.org/10.4238/2015.December.23.30
Trapero-Mozos A, Morris WL, Ducreux LJM et al (2018) Engineering heat tolerance in potato by temperature-dependent expression of a specific allele of HEAT-SHOCK COGNATE 70. Plant Biotechnol J 16:197–207
Verardi CK, Resende MDV de, Costa RB da, Gonçalves P de S (2009) Adaptabilidade e estabilidade da produção de borracha e seleção em progênies de seringueira. Pesqui Agropecuária Bras 44:1277–1282 https://doi.org/10.1590/S0100-204X2009001000010
Viana JMS, Faria VR, Fonseca e Silva F, de Resende MDV (2011) Best linear unbiased prediction and family selection in crop species. Crop Sci 51:2371–2381. https://doi.org/10.2135/cropsci2011.03.0153
Viana JMS, Faria VR, Fonseca e Silva F, de Resende MDV (2012) Combined selection of progeny in crop breeding using best linear unbiased prediction. Can J Plant Sci 92:553–562. https://doi.org/10.4141/cjps2011-110
Watanabe KN, Kikuchi A, Shimazaki T, Asahina M (2011) Salt and drought stress tolerances in transgenic potatoes and wild species. Potato Res 54:319–324
Funding
This work was supported by CAPES/PROAP, Embrapa, FAPERGS (2551-0001703-0, 2019); CNPq (429368/2016-0) and its partnership with TWAS (TWAS/CNPq 154585/2017-3). GH acknowledges CNPq (314590/2020-0) for the productivity research fellowship. In addition, we acknowledge USDA Potato Gene Bank, for sending part of the studied germplasm from which were selected the accessions BGB096 (PI175404), BGB098 (PI197760), BGB101 (PI320290), BGB102 (PI320281), BGB103 (PI320286), BGB107 (PI472820), BGB109 (PI209412) and BGB113 (PI414143), as well as Dr. César Augusto Brasil Pereira Pinto, from Universidade Federal de Lavras, for sending BGB088, BGB 089, BGB091 and BGB093. We also acknowledge Antônio Costa de Oliveira (UFPel, Brazil), Arione da Silva Pereira (Embrapa, Brazil) and Paola Gaiero (UDELAR, Uruguay) for their helpful comments for the improvement of earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bashir, I., Nardino, M., Castro, C.M. et al. Genotypic Response and Selection of Potato Germplasm Under Heat Stress. Potato Res. 66, 85–104 (2023). https://doi.org/10.1007/s11540-022-09573-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-022-09573-w