Abstract
Auxin is known to be involved in various developmental processes, including meristem identity, shoot branching and initiation of potato tubers. The previously identified StYUCCA8 gene in potato that exhibits a peak in gene expression after tuber induction and prior to tuber swelling was cloned and over-expressed in order to study the effects of altered auxin content on shoot and stolon architecture and tuber development. The potato plants transformed with the 35S::StYUCCA8 construct exhibited increased shoot and stolon branching, reduced leaf size, lower average tuber fresh weight and enhanced adventitious and lateral root formation. Investigation of the IAA content revealed that the concentration of auxin was not altered in the shoot apex but was significantly lower in the basal part of the stem despite the several 100-fold increase of expression of the StYUCCA8 gene in three independent transgenic clones. This is the first time a potato YUCCA gene is used in an experiment in order to identify the role of endogenous auxin biosynthesis in potato plant development. Our research helps elucidate the importance of small changes of auxin content on several developmental events of the potato plant, such as shoot, stolon and root architecture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Auxin is the most extensively studied plant hormone, with effects on various aspects of plant development, including shoot branching (Domagalska and Leyser 2011), flowering (Cheng et al. 2006), lateral root initiation (Dubrovsky et al. 2008), root gravitropic response (Muller et al. 1998) and embryo patterning (Vieten et al. 2005). Auxin metabolism and auxin transport are the two main determinants that control auxin content in the plant cell. Auxin distribution occurs by diffusion and/or by active transport involving influx and efflux carriers. The transport of auxin into plant cells is mediated by the influx carriers such as the AUX1-LAX gene family (Marchant et al. 1999, 2002), while polarity determining efflux carriers have been identified as the PIN family of proteins (Krecek et al. 2009). Influx and efflux carriers sustain the polar auxin transport (PAT) pathway (Gälweiler et al. 1998) that distributes auxin from the sites of biosynthesis to other tissues of the plant where auxin participates in developmental processes, such as lateral root formation (Marchant et al. 2002) or gravitropic response (Muller et al. 1998). In Arabidopsis, it has been demonstrated that several tissues such as young leaves, cotyledons and roots have the capacity to produce auxin (Ljung et al. 2001). Two major auxin biosynthesis pathways have been proposed, a tryptophan(Trp)-dependent (Zhao et al. 2001) and a Trp-independent pathway (Normanly et al. 1993). Indole-3-glycerol phosphate or indole is the likely precursor of IAA in the Trp-independent pathway, but the complete pathway has not been elucidated yet (Nonhebel 2015). Several pathways have been proposed for Trp-dependent biosynthesis: the indole-3-acetamine (IAM) pathway, the indole-3-pyruvic acid (IPA) pathway, the tryptamine (TAM) pathway and the indole-3-acetaldoxime (IAOx) pathway (reviewed in Lehmann et al. 2010). These pathways have not yet been fully elucidated, but several genes have been identified. For example, the members of the YUCCA-gene family (flavin mono-oxygenase) were shown to catalyse the last step of the IPA pathway, converting IPA into IAA (Mashiguchi et al. 2011) as well as catalysing the hydroxylation of the amino group of tryptamine in the TAM pathway, a rate-limiting step in auxin biosynthesis (Zhao et al. 2001) (auxin biosynthesis pathways and genes involved in these pathways reviewed in Mano and Nemoto 2012).The main sites of auxin biosynthesis are mainly apical meristems and the young leaves (Ljung et al. 2001). Auxin transport from the sites of biosynthesis to the lower parts of the plant mediates shoot branching (Prusinkiewicz et al. 2009). There are two main hypotheses on how auxin transport regulates shoot branching. In the canalization-based model, auxin is transported basipetally from the site of biosynthesis to the lower parts of the plant in part mediated by PIN proteins (Vieten et al. 2005). In this model, the release of axillary bud dormancy requires the transport of locally produced auxin into the main shoot in which strigolactones (SLs) play an inhibitory role (Prusinkiewicz et al. 2009). According to the second messenger model, auxin in the shoot mediates the production of a second messenger that has a direct effect on bud outgrowth (Snow 1937; Sachs and Thimann 1967). Cytokinins and strigolactones have both been found to have a role in bud outgrowth. When cytokinin (kinetin) is applied to lateral buds, they are released from inhibition (Sachs et al. 1967). Strigolactones on the other hand are found to have an inhibitory role on shoot branching (Gomez-Roldan et al. 2008). The biosynthesis of cytokinins (Nordström et al. 2004; Tanaka et al. 2006) and strigolactones (Brewer et al. 2009; Hayward et al. 2009) have been found to be regulated by auxin, making both cytokinins and strigolactones good candidates for the role of secondary messenger.
In potato, stolons are underground diageotropically growing shoots that, under environmentally favourable conditions, develop tubers at their apical meristems (Ewing and Struik 1992). Even though the relationship between stolon branching and the number of tubers produced is not extensively studied, it has been shown that the degree of stolon branching is one of the factors affecting the number and size of the tubers (Celis‐Gamboa et al. 2003). In Roumeliotis et al. 2012), we showed that auxin biosynthesis is involved in stolon branching and tuber development and that the branching mechanism in the stolon seems to be similar to the auxin-mediated mechanism that mediates branching in shoots. In vitro application of auxin to single-nodal potato explants resulted in an earlier tuberisation phenotype with sessile and slightly smaller tubers (Xu et al. 1998). In vivo, we have found that, in the stolon tips, there was a peak in auxin content a few days after induction to tuberise and prior to visible swelling. A role for auxin in the transition from stolon to tuber is accompanied by increased transcription levels of a YUCCA homolog in potato, StYUC-like1 (hereafter mentioned as StYUCCA8) and the StPIN family of genes during early stages of tuberisation (Roumeliotis et al. 2012). Several attempts have been made to elucidate the role of auxin in tuber initiation, formation and yield by manipulating the endogenous auxin content. Overexpressing known auxin biosynthesis-related genes from Arabidopsis (Kim et al. 2013) or Pseudomonas syringae (Spena et al. 1991) and tuber-specific overexpression of tms1 gene from Agrobacterium (Kolachevskaya et al. 2015) have provided contradictory results for several characteristics such as tuber yield, tuber number, tuber size and overall potato plant architecture. Still, no potato native auxin biosynthesis genes have been used so far in similar studies. The fact that StYUCCA8 exhibits a peak in expression during the early stages in tuber development renders this gene the best candidate to investigate the role of endogenous auxin in tuber initiation.
Here, we describe the analysis of transgenic potato plants over-expressing a potato native auxin biosynthesis gene, StYUCCA8. We report altered IAA content and alterations in several developmental processes, including root development, shoot and stolon branching and tuber formation. Our results provide new insight on how auxin content can be altered in various tissues when overexpressing a native YUCCA gene in potatoes and the effect of this alteration on a series of developmental characteristics including number of tubers and tuber size.
Materials and Methods
Identification of StYUCCA-Like Gene Sequences
Sixteen predicted candidate potato YUCCA genes based on automated sequence homology (2016 annotations) were used in BLAST queries against the Potato Genome Browser (http://solanaceae.plantbiology.msu.edu/) to identify all possible transcripts (gene names, transcript numbers and accession numbers given in Supplementary Figs 1 and 2). All AtYUCCAs and 13 predicted StYUCCAs with complete matching transcripts were examined for sequence homology in a phylogenetic analysis (phylogenetic tree given in Supplementary Fig. 1). StYUC-like1 gene name (Roumeliotis et al. 2012) was updated to StYUCCA8 following the NCBI-predicted gene name.
RNA-seq Data for the StYUCCA-Like Genes
The potato genome browser (PGSC 2011) was used to retrieve RNA-seq data of different tissues including shoot apex, flower, leaves, petiole, stem, stolon, tuber and root tissue of the RH genotype (Zhou et al. 2020). Expression levels for all transcript numbers identified were estimated using the number of fragments per kb per million reads (or FPKM). Only transcripts that exhibited RFPK higher than 0.2 for at least two different samples were used in the results table (for a complete table for all transcript numbers, please check Supplementary Fig. 2).
StYUCCA8 Cloning and Plant Transformation
Gateway® technology was used for the cloning of StYUCCA8 (GeneBank accession number JN935396) from Solanum tuberosum group Andigena genomic DNA. The plasmid pK7WG2 (Karimi et al., 2002) was used as the destination vector for the over-expression of the gene under the CaMV35S promoter (CaMV 35S::StYUCCA8). Agrobacterium (AGl0)-mediated plant transformation was performed on stem cuttings as described in (Visser et al. 1989). A total of 12 transgenic clones were obtained. Three transgenic clones considered to be representative for all 12 different transgenic clones, based on the aboveground branching phenotype, shoot length and on the StYUCCA8 transcript levels, were used for more detailed studies.
Plant Materials and Measurement of Auxin Concentration
Single-node cuttings from short-day-grown potato plants (Solanum tuberosum group Andigena) transformed with the CaMV 35S::YUCCA8 construct were propagated in vitro, on standard Murashige and Skoog medium (Murashige and Skoog 1962) 2% (w/v) sucrose. Potato plants were propagated in vitro and grown for 4 weeks before being transferred to soil-filled pots in the greenhouses of Unifarm (Wageningen University and Research). After 5 weeks, the shoot apex and basal internode stem segments were harvested and immediately frozen in liquid nitrogen. For all tissue samples, two biological repeats were collected and tissues from three different plants were pooled for each repeat. All samples were ground to a fine powder and stored at − 80 °C. The auxin extraction was performed as described in (Roumeliotis et al. 2012). The LC–MS/MS analysis, on the same tissue samples, was performed as described in (Ruyter-Spira et al. 2010).
Quantitative RT-PCR
The same material as used for auxin measurements was used for qRT-PCR. RNA was extracted using the Qiagen RNaesy Plant mini kit and DNAse I-treated. cDNA synthesis was performed using the Bio-Rad iScript cDNA synthesis kit, and qRT-PCR was performed using the Bio-Rad cycler. The eIF3e gene was used as a reference gene (primer sequence in Supplementary Fig. 3). Data analysis was performed using the Bio-Rad iQ5 software.
Assessment of the Branching Phenotype
Potato plantlets grown in vitro for 2 weeks were placed in 5-L pots with soil. The height and the branching phenotype of the plants were monitored weekly. All side shoots longer than 5 cm were considered to be branches, and their length was measured. After 8 weeks, tissue samples were harvested (shoot apex) to estimate the expression of the StYUCCA8 gene in the wild-type plants and in the transgenic plants. After 10 weeks, plants were transferred to a climate chamber with 8 h light (18ºC) and 16 h dark (14ºC) to induce tuberisation, and 17 days later, plants were harvested (both above and below ground parts). Tubers with a size larger than 0.5-cm diameter were collected and weighed per plant to estimate fresh weight. For all the statistical analysis, we used SPSS Statistics v19 (SPSS©, Inc., 2001, Chicago, IL), post hoc comparisons LSD, a = 0.05.
Growth Conditions for Estimating Changes in Root Architecture
35S:StYUCCA8 over-expression transgenic and untransformed control plants were grown in vitro for 4 weeks before being transferred to an aeroponic system (Nutricolture co. UK) on Hoagland’s solution. Root architecture was monitored daily for length and branching.
Results
Phylogenetic Tree Analysis of the AtYUCCAs and StYUCCA-Like Genes
According to Kim et al. (2013), eight YUCCA genes were identified in the potato genome. A more recent annotation of YUCCA like genes in NCBI revealed 16 predicted candidates based on automated sequence homology (2016 annotations).
Thirteen out of 16 predicted potato YUCCA-like genes returned highly similar results when used in the BLAST query against the potato RNA-seq transcripts (Supplementary Figs. 1 and 2). Three accession numbers out of 16 had only partially matching transcripts (XM_015312507.1) or did not return significantly similar results (XM_015304844.1, XM_015306951.1) and were not used further in this study.
All predicted potato YUCCA genes were allocated in two branches of the phylogenetic tree. Each of those two branches contains Arabidopsis and potato predicted YUCCA genes. The first branch contains AtYUCCA1, 4, 7, 3 and 9 from Arabidopsis and potato-predicted StYUCCA4, 5b, 5a, 7, 8 and 3 genes. The second branch contains AtYUCCA10 and StYUCCA10a to StYUCCA10g. A third branch is formed with AtYUCCA2 and 6 and contains no potato-predicted YUCCA gene (Supplementary Fig. 1).
Expression Pattern of the StYUCCA-Like Genes
Five accession numbers (corresponding to predicted Gene names StYUCCA3, 4, 5a, 7 and 8) returned highly similar transcripts that exhibited RPKM values in more than one sample (Fig. 1 and Supplementary Fig. 2). Based on the RPKM values, the most highly expressed YUCCA-like gene is StYUCCA7. Transcripts of this gene were found in all samples, with the highest value present in the flower sample. StYUCCA7 RFPK value for the flower is the highest value in the table across all genes and all samples. StYUCCA3, 4, 5a and 8 are found to be present in the majority of the samples, but in lower abundance compared to StYUCCA7 with few exceptions.
Heatmap of expression of the StYUC-like genes in the corresponding tissues according to the RNA-seq data of the potato diploid genotype RH. Expression levels in the various tissues are indicated by shades of red, where white indicates no expression detected. Numbers in the cells correspond to the RFPK values of each gene (third column from left) for the corresponding sample (top line). Only YUCCA genes that exhibited high RPKM values in more than one sample are included. Transcript id numbers correspond to the BLAST results of the corresponding StYUC-like gene against PGSC_DM_v3.4_transcript-update.fasta using the http://solanaceae.plantbiology.msu.edu blast tool
Out of the five genes that were found to be expressed in the young tuber, StYUCCA8 exhibits the highest expression. In the stage of mature tuber, StYUCCA8 expression seems to be not detected. This is an interesting result because it verifies the previously reported peak in expression of the StYUCCA8 gene in the stages of tuber induction and prior to visible swelling (Roumeliotis et al. 2012) establishing this gene as the main candidate to study the role of local auxin biosynthesis in stolon and tuber development.
Plants Over-expressing StYUCCA8 Exhibit Reduced Apical Dominance, Longer Side Shoots and Smaller Leaf Size
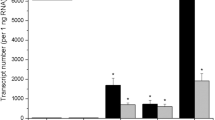
In order to analyze the function of the StYUCCA8 gene in potatoes, we made a construct containing the entire coding region of the StYUCCA8 gene and placed it under the control of a constitutive CaMV 35S promoter. Investigation of the transcript levels of the StYUCCA8 gene in the apex of the transgenic clones revealed a several 100-fold increase in the transcript levels compared to the control (Fig. 2). Three transgenic clones considered to be representative for all 12 different transgenic clones based on the above ground branching phenotype, shoot length and on the StYUCCA8 transcript levels were grown under greenhouse conditions. The transgenic lines were monitored weekly for their height and overall above-ground phenotype to identify possible differences between the transgenic plants and the untransformed control plants. After 12 weeks, no statistically significant differences in height were detected between the transgenic clones and the non-transgenic controls (Fig. 3a). All three clones exhibited extensive branching (Figs. 3b and 4a) and smaller leaf size (Fig. 4b). While the control plants had an average of three branches per plant, all three transgenic clones had, on average, more than five branches (Fig. 3b). Examination of the length of the branches in each node revealed the transgenic clones exhibited significantly longer branch lengths for almost all higher branches (Fig. 3c) except for the first basal branch. The control plants had an average length of 43, 30 and 17 cm for branches one to three, respectively, and no side branches above the fourth node. In contrast, for clone 8 the average length of branches one to six were 38, 21, 30, 23, 32 and 31 cm, respectively, and similar results were found for the other transgenic clones. These results show that the transgenic clones exhibited increased branching, and that for branches 2 to 6, the length of the respective branch is longer in comparison to the controls.
Fold increase of the expression of the StYUCCA8 gene in the apex in four transgenic clones and the untransformed control grown in the greenhouse. The StYUCCA8 gene expression in the untransformed control is set to one fold. Error bars represent standard error for two biological repeats with three plants pooled per biological repeat
A Height measurements for three transgenic clones and the untransformed control over a period of 12 weeks of growth in the greenhouse. Error bars represent standard error for ten individual plants per clone. Dotted clone indicates the early stage when differences in height between three clones and the untransformed control are statistically significant. Significant differences (AVOVA test, a = 0.05) 2.5 weeks after the plants were placed in the greenhouse are represented with a star. B Scoring of the branching frequency for three transgenic clones and the untransformed control. C Scoring of the length of the branches for three transgenic clones and the untransformed control. Error bars represent standard error for two biological repeats with three plants pooled per repeat and stars indicate statistical significant differences between the transgenic clones and the untransformed control (ANOVA test, a = 0.05)
A Comparison of the branching phenotype between a transgenic clone and the untransformed control with a diagrammatic representation of the branching phenotype. Red arrows indicate the branches on the transgenic clone. B Comparison between the leaf size of the untransformed control and transgenic clones 8, 9 and 12
Plants Over-expressing StYUCCA8 Exhibit Higher Stolon Branching, Increased Number of Tubers per Plant and Smaller Tuber Size
To investigate the effect of StYUCCA8 over-expression on stolons and tuber formation, transgenic plants grown in the greenhouse for 10 weeks under non-inductive long-day conditions were transferred to short-day conditions to induce tuberization (see the ‘Materials and methods’ section). After 2 weeks in the growth chamber, clone 8 showed numerous buds and sessile tuber-initials on almost all side buds indicating increased stolon branching (Fig. 5a). For the estimation of final tuber numbers (Fig. 6a), small tuber-initials were not included if smaller than 0.5 cm. For all three transgenic clones, the average tuber weight was significantly lower compared to the control and the number of tubers was increased for clones 9 and 12 compared to the wild-type plants (Fig. 6c). Clones 9 and 12 had a significantly higher number of tubers, however, with the same total fresh weight as the untransformed control (Fig. 6b).
A Scoring of the average number of tubers per plant for each clone. B Scoring of the average tuber fresh weight per plant for each clone. C Scoring of the average tuber weight for each transgenic clone and the untransformed control. Error bars are standard error for 6 plants per clone, and stars indicate statistically significant differences between the clone and the untransformed control (ANOVA test, a = 0.05)
Plants over-expressing StYUCCA8 exhibit short bushier roots
Preliminary observations on the root architecture of plants grown in soil indicated a bushy root structure for the 35S::StYUCCA8 transgenic plants compared to the untransformed controls; however, the differences were difficult to quantify in a soil-grown system. To be able to score for altered root morphology in a non-destructive way, transgenic and control plants were grown in the aeroponic system previously described in (Roumeliotis et al. 2012). It was evident from our observations that the roots of the transgenic lines exhibited increased adventitious rooting and lateral root formation compared to the untransformed control plants, manifesting a bushier root phenotype (Fig. 5b) in alignment with the initial observations of plants grown in pots with soil. The increased capacity of lateral root formation was evenly distributed over the entire root system established by the explants.
Transformed Plants with the 35S::YUCCA8 Construct have Lower Auxin Content in the Basal Stem
To investigate the effect of the 35::StYUCCA8 construct in the transgenic potato clones on the auxin content, we assayed auxin levels in the apex and in the basal part of the stem in mature plants grown in the greenhouse (Fig. 7). Estimation of the IAA content in the apex where auxin biosynthesis is predominately taking place showed no significant differences between the 35S::StYUCCA8 clones compared and the untransformed controls (190 to 260 pmol/g FW for the transgenic clones and 210 pmol/g FW for the untransformed control; Fig. 7a). However, in the basal part of the stem, the 35S::StYUCCA8 transgenic clones have less free auxin compared to the untransformed control plants (Fig. 7b). The untransformed control plants have an average IAA content of 130 pmol, while in the transformed clones, IAA content ranges from 62 to 83 pmol. These results are contrary to expectations in plants where a higher level of IAA is anticipated due to the overexpression of a gene participating in auxin biosynthesis.
IAA content for 3 independent transgenic clones and the untransformed control in the apex (left) and basal stem (right). Plants were grown in the greenhouse for 5 weeks prior to harvesting the tissues. Error bars represent standard error for two biological repeats with three plants pooled per repeat and stars indicate statistical significant differences (ANOVA test, a = 0.05)
Discussion
In this study, we investigated the expression pattern for all predicted YUCCA-like genes in potatoes that have been annotated in NCBI. Five out of 16 predicted YUCCA genes (genes StYUCCA3, 4, 5a, 7 and 8) were found to have significant similarities to transcripts that are expressed in various degrees in almost all tissue samples; therefore, these genes are expected to be expressed in almost all parts of the plant.
The most highly expressed YUCCA-like gene in the majority of the samples (flower, leaf, petiole, stem and root sample) is StYUCCA7, with the highest expression being noticed in the flower sample. StYUCCA3, StYUCCA4 and StYUCCA5a also exhibit an expression level that varies among tissues with a degree of tissue preference. StYUCCA8 seems to exhibit a peak in expression in young tuber tissue and lower expression in petiole, stem, stolon, shoot apex and leaf.
Auxin has been found to have a role in tuber initiation with a peak in auxin content being noticed after tuber induction and prior to the initiation of tuber swelling (Roumeliotis et al. 2012); therefore, investigation of the auxin biosynthesis YUCCA-like genes in the stolon and in the young tuber becomes important. StYUCCA3, 4, 5a, 7 and 8 were found to be expressed in the stolon and in the young tuber. StYUCCA3, 4, 5a and 8 seem to have higher expression in the young tuber sample compared to the stolon sample, with the highest expression being noticed for StYUCC8. The StYUCC8 expression pattern is in accordance with the previously described peak in expression and increase in auxin content during tuber initiation prior to tuber swelling (Roumeliotis et al. 2012). Among all StYUCCA-like genes, StYUCCA8 is predominantly expressed in the young tuber, while its expression seems to be completely shut down at the mature tuber stage. These results indicate that for all StYUCCA-like genes, expression exhibits some spatial distribution suggesting that different StYUCCA genes contribute to auxin content locally in the different plant tissues. In Chen et al. (2014), auxin overproduction in shoots fails to totally rescue auxin deficiency phenotype in the roots of Arabidopsis, meaning that locally produced auxin is required for normal root development. The spatial distribution on StYUCCA gene expression we observed could indicate that different StYUCCA genes contribute to the local levels of auxin content that is important for tissue and meristem identity in potato, suggesting StYUCCA8 to be the predominant contributor to auxin biosynthesis in the young tuber and establishing StYUCCA8 as the main native potato candidate gene to study the role of auxin in stolon and tuber development. Based on these results, StYUCCA8 gene was cloned and its role in potato plant development and tuber initiation was further investigated.
In order to investigate the effect of StYUCCA8 in potato plant and tuber development, StYUCCA8 gene was cloned and recombined into Gateway vector pK7WG2 under the control of the 35S promoter (35S::StYUCCA8) and stable transformed potato plants were created. Independent transgenic clones with the 35S::StYUCCA8 construct exhibited decreased apical dominance in the shoot and stolon, smaller leaf size and enhanced lateral root formation. Several of these findings are indicative of decreased IAA levels in the stem, which is in contrast to an expected increase of IAA content in the StYUCCA8 overexpressing lines. Expression analysis of the StYUCCA8 transgene was performed in order to investigate whether the StYUCCA8 gene was truly expressed in high levels. The results showed that the StYUCCA8 was indeed expressed several hundred times fold higher compared to the control (Fig. 2). Therefore, estimation of the auxin content in the tissues was necessary in order to understand the origin of the phenotypes that resembled auxin deficiency. Assessment of the IAA content in the shoot apex and basal stem revealed no differences for auxin concentrations in the apex, but IAA content in the basal stem was decreased in the transgenic clones compared to the untransformed control (Fig. 7). Shoot branching and apical dominance in stems are mediated through polar auxin transport from the sites of biosynthesis to the lower parts of the plant. Lower levels of auxin in the basal stem are expected to result in decreased apical dominance and increased shoot branching (Prusinkiewicz et al. 2009); therefore, lower auxin content in the basal shoot is in accordance with the decreased apical dominance phenotype that was observed. In all three analysed transgenic clones, the number of shoot branches was increased from the third node and up and their length was significantly longer compared to the untransformed control due to the earlier release of the buds into growth. In addition to increased shoot branching, stolons also exhibited decreased apical dominance, resulting in increased stolon branching and higher number of tubers and tuber initials. In Roumeliotis et al. (2012), we presented evidence that the mechanism that mediates stolon branching is homologous to the mechanism that regulates shoot branching. Therefore, lower auxin content in the basal shoot could also provide a possible explanation for the decreased apical dominance noticed in the stolons. A decreased apical dominance in the stolons releases the stolon buds from dormancy allowing the development of secondary stolons that can develop into tuber-initials under tuber inductive conditions, resulting in a larger number of tubers.
Attenuating auxin biosynthesis by production of double, triple and quadruple yuc knockout lines in Arabidopsis, has been shown to result in decreased leaf size (Cheng et al. 2006). This links auxin biosynthesis with leaf size. Thus, the reduced leaf size observed in the 35S::StYUCCA8 plants is consistent with lower auxin levels in the stem.
Auxin content and local auxin biosynthesis are known to be important for root development and lateral root formation (Dubrovsky et al. 2008; Zhao 2018). In (Dubrovsky et al. 2008), auxin-related activation is necessary for founder cell differentiation, the first step in lateral root formation. Therefore, altering the expression of a YUCCA gene would be expected to have an impact on root development. In all three transgenic clones overexpressing StYUCCA8, increased lateral root formation was observed, which is in accordance with the expected higher auxin content due to the StYUCCA8 overexpression.
It is interesting to notice that overexpressing the native StYUCCA in potato resulted in a number of contradictory phenotypes. Overexpressing StYUCCA8 was expected to result in higher auxin content in the whole plant. What we observed in the stem and stolons was reduced apical dominance and smaller leaf size. Measuring the auxin content revealed that auxin content was either not altered (apex) or lower (basal stem) than the control. In contrast, increased lateral root formation is a typical high auxin phenotype. The high transcript levels of StYUCCA8 that were measured in the transgenic lines suggest that co-suppression is unlikely. In Stepanova et al. (2011), YUCCA overexpression did not lead to significantly higher levels of auxin due to absence of substrate for the YUC protein. In addition, in Takato et al. (2017), a feedback mechanism is described that regulates auxin biosynthesis by controlling StYUCCA genes expression through the Aux/IAA and SCFTIR1/AFB-mediated auxin-signalling pathways. It is possible that overexpressing the native StYUCCA8 gene in potato resulted in activating a feedback regulation mechanism for auxin biosynthesis, which limited the StYUCCA8 substrate in order to avoid excessive auxin biosynthesis, suggesting that auxin biosynthesis and auxin content can be subject to strict control by feedback mechanisms. The fact that in the roots we notice increased lateral root formation, a typical high auxin phenotype, suggests that this feedback mechanisms might be tissue related.
Tuber fresh weight of the StYUCCA8 transgenic lines was either not different or lower compared to the control (Fig. 6b). This result, in combination with the increased number of tubers produced by the transgenic lines, resulted in a smaller average tuber weight (Fig. 6c). As discussed above, the increased number of tubers could be a result of the decreased apical dominance in the stolons under tuber inductive conditions. The increased number of tubers would distribute the yield to more tubers, resulting in smaller individual tubers. A possible impact for auxin biosynthesis in potato tuber yield has been investigated in several publications and auxin seems to be established as an important regulator of tuber development (a review of auxin as a regulator of tuber development in Kondhare et al., 2021). In this concept, several attempts have been made to investigate the role of endogenous auxin in tuber and potato plant development using a GMO approach. The earliest experiment to alter endogenous auxin content using a GMO approach was by overexpressing the iaaL gene in potato to increase the levels of Lysine conjugated auxin, therefore limiting the levels of active auxin (Spena et al. 1991). The transgenic potato plants overexpressing the iaaL gene exhibited elongated internodes, epinastic bending of older leaves, less developed roots and a greater number of smaller tubers and increased the crop yield (Spena et al. 1991; Fladung 1993). In Ivana et al. (1997), overexpression of the cytokinin biosynthesis ipt gene resulted in increased cytokinin and auxin content. The altered CKs/auxin ratio resulted in a different response of the transgenic plants to tuber inductive stimuli but did not provide a clear answer on the role of auxin itself. 35S-driven overexpression of a AtYUCCA gene in potato resulted in decreased tuber number and decreased tuber yield (Kim et al. 2013). Transgenic plants had narrow downward-curled leaves, elongated internodes and erect stature resembling some of the phenotypes reported in (Spena et al. 1991). In addition, the transgenic plants differed from wild type control plants in longevity and were more resistant to drought. Auxin content was not scored in Kim et al. (2013), concluding that auxin content was increased based on the phenotypes observed. A more precise approach was used in Kolachevskaya et al. (2015) where the B33-promoter of the patatin class I gene was used to drive the agrobacterium auxin synthesis gene tms1. Tubers of the transgenic plants had an increased auxin content. Potato plants exhibited earlier tuberization and increased sensitivity to sugar-induced tuberization, and the number and average weight of tubers were increased. Based on the above publications, auxin content in the whole plant seems to have a negative (Spena et al. 1991; Kim et al. 2013) or no (this publication) correlation with yield while local increase of auxin content in the tuber seems to have a positive correlation with yield (Kolachevskaya et al. 2015). It is possible that, as described for tuber initiation, local auxin biosynthesis and local auxin content in the tuber have to exhibit specific spatiotemporal distribution in order to exert a role in yield. More experiments have to be performed in this direction in order to enlighten a possible role for auxin in potato tuber yield.
In this research, we present data concerning the effect of a 35S::StYUCCA8 construct on various developmental events in potato. Overexpression of StYUCCA8 gene resulted in decreased IAA content in the basal stem, which enhanced shoot and stolon branching, increased lateral root formation and reduced leaf size. Total yield was not altered but the average tuber weight was smaller. Our research sheds light on the effect that small changes in IAA content have in several developmental events of the potato plant and on our understanding of the mechanisms that mediate branching in shoots and stolons.
Data Availability
The data of the current study are available from the corresponding authors on reasonable request.
References
Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physioliology 150:482–493
Celis-Gamboa C, Struik PC, Jacobsen E, Visser RGF (2003) Temporal dynamics of tuber formation and related processes in a crossing population of potato (Solanum tuberosum). Ann Appl Biol 143:175–186
Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y (2014) Auxin overproduction in shoots cannot rescue auxin deficiencies in arabidopsis roots. Plant Cell Physiol 55:1072–1079
Cheng YF, Dai XH, Zhao YD (2006) Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev 20:1790–1799
Domagalska MA, Leyser O (2011) Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol 12:211–221
Dubrovsky JG, Sauer M, Napsucialy-Mendivil S, Ivanchenko MG, Friml J, Shishkova S, Celenza J, Benková E (2008) Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. Proc Natl Acad Sci U S A 105:8790–8794
Ewing EE, Struik PC (1992) Tuber formation in potato: induction, initiation, and growth. Hortic Rev 14:89–133
Fladung M (1993) Influence of the indoleacetic acid-lysine synthetase gene (iaaL) of Pseudomonas syringae subsp. savastanoi on yield attributes of potatoes. Plant Breed 111:242–245
Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282:2226–2230
Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pages V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC et al (2008) Strigolactone inhibition of shoot branching. Nature 455:189–194
Hayward A, Stirnberg P, Beveridge C, Leyser O (2009) interactions between auxin and strigolactone in shoot branching control. Plant Physiol 151:400–412
Ivana M, Lidiya S, Miloš O, Oksana Z, Tatyana K, Josef E, Jaroslava O, Svetlana G, Yurii R, Nina A (1997) Growth pattern, tuber formation and hormonal balance in in vitro potato plants carrying ipt gene. Plant Growth Regul 21:27–36
Kim JI, Baek D, Park HC, Chun HJ, Oh D-H, Lee MK, Cha J-Y, Kim W-Y, Kim MC, Chung WS et al (2013) Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol Plant 6:337–349
Kolachevskaya OO, Alekseeva VV, Sergeeva LI, Rukavtsova EB, Getman IA, Vreugdenhil D, Buryanov YI, Romanov GA (2015) Expression of auxin synthesis gene tms1 under control of tuber-specific promoter enhances potato tuberization in vitro. J Integr Plant Biol 57:734–744
Krecek P, Skupa P, Libus J, Naramoto S, Tejos R, Friml J, Zazimalova E (2009) The PIN FORMED PIN protein family of auxin transporters. Genome Biol 10:249.01-249.11
Lehmann T, Hoffmann M, Hentrich M, Pollmann S (2010) Indole-3-acetamide-dependent auxin biosynthesis: a widely distributed way of indole-3-acetic acid production? Eur J Cell Biol 89:895–905
Ljung K, Bhalerao RP, Sandberg G (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28:465–474
Mano Y, Nemoto K (2012) The pathway of auxin biosynthesis in plants. J Exp Bot 63:2853–2872
Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18:2066–2073
Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell Online 14:589–597
Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, Hanada A, Yaeno T, Shirasu K, Yao H et al (2011) The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci 108:18512–18517
Muller A, Guan C, Galweiler L, Tanzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J 17:6903–6911
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nonhebel HM (2015) tryptophan-independent indole-3-acetic acid synthesis: critical evaluation of the evidence. Plant Physiol 169:1001–1005
Nordström A, Tarkowski P, Tarkowska D, Norbaek R, Åstot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proc Natl Acad Sci U S A 101:8039–8044
Normanly J, Cohen JD, Fink GR (1993) Arabidopsis thaliana auxotrophs reveal a tryptophan-independent biosynthetic pathway for indole-3-acetic acid. Proc Natl Acad Sci 90:10355
PGSC (2011) Genome sequence and analysis of the tuber crop potato. Nature 475:189–195
Prusinkiewicz P, Crawford S, Smith RS, Ljung K, Bennett T, Ongaro V, Leyser O (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci 106:17431–17436
Roumeliotis E, Kloosterman B, Oortwijn M, Kohlen W, Bouwmeester HJ, Visser RG, Bachem CW (2012) The effects of auxin and strigolactones on tuber initiation and stolon architecture in potato. J Exp Bot 63:4539–4547
Ruyter-spira C, Kohlen W, Charnikhova T, van Zeijl A, van Bezouwen L, de Ruijter N, Cardoso C, Lopez-Raez JA, Matusova R, Bours R et al (2010) Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another below-ground role for strigolactones? Plant Physiol 155:721–734
Sachs T, Thimann KV (1967) The role of auxins and cytokinins in the release of buds from dominance. Am J Bot 54:136–144
Snow R (1937) On the nature of correlative inhibition. New Phytol 36:283–300
Spena A, Prinsen E, Fladung M, Schulze SC, Van Onckelen H (1991) The indoleacetic acid-lysine synthetase gene of Pseudomonas syringae subsp. savastanoi induces developmental alterations in transgenic tobacco and potato plants. Mol Gen Genet MGG 227:205–212
Stepanova AN, Yun J, Robles LM, Novak O, He W, Guo H, Ljung K, Alonso JM (2011) The Arabidopsis YUCCA1 flavin monooxygenase functions in the indole-3-pyruvic acid branch of auxin biosynthesis. Plant Cell 23:3961–3973
Takato S, Kakei Y, Mitsui M, Ishida Y, Suzuki M, Yamazaki C, Hayashi K, Ishii T, Nakamura A, Soeno K et al (2017) Auxin signaling through SCFTIR1/AFBs mediates feedback regulation of IAA biosynthesis. Biosci Biotechnol Biochem 81:1320–1326
Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H (2006) Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance. Plant J 45:1028–1036
Vieten A, Vanneste S, Wiśniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132:4521–4531
Visser RGF, Jacobsen E, Hesseling-Meinders A, Schans MJ, Witholt B, Feenstra WJ (1989) Transformation of homozygous diploid potato with an Agrobacterium tumefaciens binary vector system by adventitious shoot regeneration on leaf and stem explants. Plant Mol Biol 12:329–337
Xu X, van Lammeren AA, Vermeer E, Vreugdenhil D (1998) The role of gibberellin, abscisic acid, and sucrose in the regulation of potato tuber formation in vitro. Plant Physiol 117:575–584
Zhao Y (2018) Essential roles of local auxin biosynthesis in plant development and in adaptation to environmental changes. Annu Rev Plant Biol 69:417–435
Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291:306–309
Acknowledgements
We thank Tatsiana Charnikhova, Francel Verstappen, and Wendy Jo-Chen Liao for their technical support. A part of this work was done in the framework of the EU-SOL project (PL 016214-2 EU-SOL) and co-financed by The Netherlands Organization for Scientific Research (NWO; VICI grant, 865.06.002 and Equipment grant, 834.08.001 to H.B.). E.R. gratefully acknowledges the financial support from the Bakalas Foundation and the Stichting Veenhuizen-Tulp fonds.
Funding
Part of this work was done in the framework of the EU-SOL project (PL 016214–2 EU-SOL) and co-financed by The Netherlands Organization for Scientific Research (NWO; VICI grant, 865.06.002 and Equipment grant, 834.08.001 to H.B.). E.R. gratefully acknowledges the financial support from the Bakalas Foundation and the Stichting Veenhuizen-Tulp fonds.
Author information
Authors and Affiliations
Contributions
E.R. (first author) is the primary investigator and responsible for drafting the paper. C.W.B.B. (project co-supervisor) and R.G.F.V. (project supervisor) were involved in the review and writing of the manuscript. All other authors were involved in the writing process at different stages, and their names are ordered by magnitude of contribution from higher to lower.
Corresponding author
Ethics declarations
Ethics Approval
n/a (no tests on animals, human cell lines etc.)
Consent to Participate
n/a.
Consent for Publication
All authors are aware of this manuscript and its content and consent for publication.
Code Availability
n/a.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roumeliotis, E., Kloosterman, B., Oortwijn, M. et al. Over-expression of a YUCCA-Like Gene Results in Altered Shoot and Stolon Branching and Reduced Potato Tuber Size. Potato Res. 66, 67–84 (2023). https://doi.org/10.1007/s11540-022-09572-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11540-022-09572-x