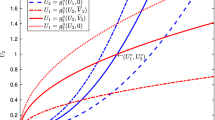
Abstract
The properties of competition models where all individuals are identical are relatively well-understood; however, juveniles and adults can experience or generate competition differently. We study here less well-known structured competition models in discrete time that allow multiple life history parameters to depend on adult or juvenile population densities. A numerical study with Ricker density-dependence suggested that when competition coefficients acting on juvenile survival and fertility reflect opposite competitive hierarchies, stage structure could foster coexistence. We revisit and expand those results. First, through a Beverton–Holt two-species juvenile-adult model, we confirm that these findings do not depend on the specifics of density-dependence or life cycles, and obtain analytical expressions explaining how this coexistence emerging from stage structure can occur. Second, we show using a community-level sensitivity analysis that such emergent coexistence is robust to perturbations of parameter values. Finally, we ask whether these results extend from two to many species, using simulations. We show that they do not, as coexistence emerging from stage structure is only seen for very similar life-history parameters. Such emergent coexistence is therefore not likely to be a key mechanism of coexistence in very diverse ecosystems, although it may contribute to explaining coexistence of certain pairs of intensely competing species.



Similar content being viewed by others
Code Availability
Computer codes written for analyses are available at GitHub: https://github.com/g-bardon/StructuredModels_Coexistence.
References
Barabás G, Meszéna G, Ostling A (2014a) Fixed point sensitivity analysis of interacting structured populations. Theor Popul Biol 92:97–106
Barabás G, Pásztor L, Meszéna G, Ostling A (2014b) Sensitivity analysis of coexistence in ecological communities: theory and application. Ecol Lett 17:1479–1494
Barabás G, Michalska-Smith MJ, Allesina S (2016) The effect of intra- and interspecific competition on coexistence in multispecies communities. Am Nat 188:E1–E12
Boutin SA (1984) The effect of conspecifics on juvenile survival and recruitment of snowshoe hares. J Anim Ecol 53(2):623–637
Caswell H (2001) Matrix population models: construction, analysis, and interpretation, 2nd edn. Sinauer Associates. Inc., Sunderland
Chesson P (2000) Mechanisms of maintenance of species diversity. Ann Rev Ecol Syst 31:343–366
Chu C, Adler PB (2015) Large niche differences emerge at the recruitment stage to stabilize grassland coexistence. Ecol Monogr 85:373–392
Cushing J (2006) Nonlinear semelparous leslie models. Math Biosci Eng 3:17–36
Cushing J (2008) Matrix models and population dynamics. Math Biol 14:47–150
Cushing J, Li J (1989) On Ebenman’s model for the dynamics of a population with competing juveniles and adults. Bull Math Biol 51:687–713
Cushing JM (1998) An introduction to structured population dynamics, volume 71. SIAM
Cushing JM, Henson SM (2012) Stable bifurcations in semelparous leslie models. J Biol Dyn 6:80–102
Cushing JM, Levarge S, Chitnis N, Henson SM (2004) Some discrete competition models and the competitive exclusion principle. J Diff Equ Appl 10:1139–1151
Cushing JM, Henson SM, Roeger L-I (2007) Coexistence of competing juvenile-adult structured populations. J Biol Dyn 1:201–231
Fujiwara M, Pfeiffer G, Boggess M, Day S, Walton J (2011) Coexistence of competing stage-structured populations. Sci Rep 1:107
Goldberg DE, Landa K (1991) Competitive effect and response: hierarchies and correlated traits in the early stages of competition. J Ecol 79:1013–1030
Haigh J, Smith JM (1972) Can there be more predators than prey? Theor Popul Biol 3:290–299
Kinlock NL (2021) Uncovering structural features that underlie coexistence in an invaded woody plant community with interaction networks at multiple life stages. J Ecol 109:384–398
Leslie P, Gower J (1958) The properties of a stochastic model for two competing species. Biometrika 45:316–330
Leslie PH (1945) On the use of matrices in certain population mathematics. Biometrika 33:183–212
Letten AD, Ke P-J, Fukami T (2017) Linking modern coexistence theory and contemporary niche theory. Ecol Monogr 87:161–177
Loreau M, Ebenhoh W (1994) Competitive exclusion and coexistence of species with complex life cycles. Theor Popul Biol 46:58–77
Meszéna G, Czibula I, Geritz S (1997) Adaptive dynamics in a 2-patch environment: a toy model for allopatric and parapatric speciation. J Biol Syst 5:265–284
Miller TE, Rudolf VH (2011) Thinking inside the box: community-level consequences of stage-structured populations. Trends Ecol Evol 26:457–466
Moll JD, Brown JS (2008) Competition and coexistence with multiple life-history stages. Am Nat 171:839–843
Neubert MG, Caswell H (2000) Density-dependent vital rates and their population dynamic consequences. J Math Biol 41:103–121
Péron G, Koons DN (2012) Integrated modeling of communities: parasitism, competition, and demographic synchrony in sympatric ducks. Ecology 93:2456–2464
Qi M, DeMalach N, Sun T, Zhang H (2021) Coexistence under hierarchical resource exploitation: the role of r*-preemption tradeoff. arXiv preprint arXiv:1908.08464
Tilman D (1982) Resource competition and community structure. (MPB-17), vol 17. Princeton University Press
Wallace MP, Temple SA (1987) Competitive interactions within and between species in a guild of avian scavengers. The Auk 104:290–295
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Ann Rev Ecol Syst 15:393–425
Wilbur HM (1980) Complex life cycles. Ann Rev Ecol Syst 11:67–93
Acknowledgements
We thank Sam Boireaud for an exploratory numerical study of some two-species models, as well as Olivier Gimenez for discussions and Coralie Picoche for comments on the manuscript. We are grateful to three reviewers for their constructive input.
Funding
Funding for Gaël Bardon’s internship was provided by the French National Research Agency through ANR Democom (ANR-16-CE02-0007) to Olivier Gimenez.
Author information
Authors and Affiliations
Contributions
GB wrote the computer code, produced the figures, and performed numerical analyses. Analytic formulas were derived by FB and GB. The first draft was written by GB with help from FB, both authors contributed equally to subsequent versions.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Invasion Analysis
We develop here the method to obtain invasion criteria in two-species two-life stages models including competition on two vital rates, such as model 1 combining competition on fertility and juvenile survival.
We first place ourselves at the exclusion equilibrium where species 1 lives without species 2:
To compute the equilibrium densities \(n_{1j}^*\) and \(n_{1a}^*\), we have to solve the following system corresponding to the model with a single species (we dropped here species-specific indices for simplicity):
with fertility f(n) and juvenile survival \(s_j(n)\) affected by intra-specific competition only, according to the relations
We now express \(n_j\) according to \(n_a\):
and then, as we have by assumption \(n_a \ne 0\) and \(n_j \ne 0\), we obtain
that leads to the polynomial of degree 2:
We denote \(C= (1-\gamma )\phi \) and \(D= \frac{\pi \gamma \phi }{1-s_a}\) and we find the solutions of the polynomial equation:
We search for a positive solution and we have \(C=(1-\gamma )\phi < 1\) which implies \(\alpha C - \alpha - \beta < 0\) assuming positive interaction coefficients (\(\alpha >0\) and \(\beta >0\)). Positivity is then equivalent to:
The equivalence is provided by the fact that the left term has its square inside the square root. We define the inherent net reproductive number as \(C+D = (1-\gamma )\phi +\frac{\pi \gamma \phi }{1-s_a}\) that must exceed 1 to have a viable species. We finally obtain the densities at a stable positive equilibrium for the model with a single species:
provided that
We have then obtained the densities at the exclusion equilibrium. We have now to evaluate the stability of the equilibrium \((n_j^*,n_a^*,0,0)\): if it is locally asymptotically stable, the excluded species cannot invade the community starting from very small density. Conversely, if it is unstable, this means that the excluded species can invade provided that equilibria of the single-species model are restricted to stable fixed points. This last restriction is important. Indeed, if the single-species model has a stable two-cycle or an even more complex fluctuating attractor, the fixed point will already be unstable, and a proper invasion analysis should instead be done on this more complex attractor. Two-cycles tend to occur for \(s_a=0\) (Cushing et al. 2007), as shown in Supplement C. So long as \(s_a>0\) this model does not show 2-cycles—so far as we have seen numerically and following the argumentation of Cushing et al. (2007) for model 2. The combination \(s_a = 0\) and \(\gamma = 1\) renders the projection matrix primitive, which in turns creates possibilities for bifurcations towards two-cycles directly from the trivial single-species (0,0) equilibrium (Cushing and Li 1989; Cushing 2006). We therefore restrict ourselve to the case \(s_a>0\), although the equations derived above in this Appendix for the single-species fixed point are also valid whenever \(s_a=0\).
To evaluate the (un)stability of the exclusion equilibria in the two-species system, we have to compute the eigenvalues of the Jacobian of the 2 species system evaluated at the exclusion equilibrium. The full system iterated over a time step reads:
We place ourselves at the abovementioned exclusion equilibrium where species 1 dominates the community and species 2 is absent. The Jacobian evaluated at this point is a \(4 \times 4\) matrix and it has the following triangular block form (see also Cushing 2008):
Therefore, we only need to know the eigenvalues of the \(2 \times 2\) matrices \(B_1\) and \(B_3\). The \(B_1\) matrix corresponds to the Jacobian of the system in the absence of species 2, so provided that the single-species equilibrium is stable, we can infer that it has a spectral radius lower than 1. Unfortunately, although all simulations that we have done, even for very large fertilities or low competition coefficients, provide a stable equilibrium for \(C+D>1\), it is not straightforward to show local stability analytically. Indeed matrix \(B_1\) writes:
with \(n_a\) given by Eq. 32, making the use of Jury conditions (used below on the simpler \(B_3\) block) difficult here, although the determinant simplifies somewhat. We therefore conjecture the stability of the single-species equilibrium, with a spectral radius of \(B_1\) below 1, which we deem reasonable in this context. The \(B_3\) matrix is given by:
The \(B_3\) matrix block, which is part of the Jacobian, also corresponds to the projection matrix of species 2 when invading species 1, which implies that the exclusion equilibrium is not stable when species 2 has a growth rate larger than 1 (i.e., the modulus of \(B_3\)’s leading eigenvalue is larger than 1). The stability of this two-dimensional system can be investigated thanks to the Jury conditions, given by the Eqs. (38, 39, 40):
with \(J(\textbf{n})\) the Jacobian matrix of the two-compartment system whose stability is being evaluated. If these three conditions hold, the system is stable. If the first condition (Eq. 38) is violated, one of the eigenvalues of \(J(\textbf{n})\) is larger than 1, which means in our context that the excluded species can invade the community. The violations of the conditions (39) and (40) correspond to the creation of limit cycles or invariant loops (Neubert and Caswell 2000). We then check these conditions on the \(B_3\) matrix. We have:
We start with the third condition (40):
This equation will always be satisfied with biologically meaningful parameters and strictly competitive interaction between species:
-
The third term of (41) is positive because each parameter is positive,
-
\((1-\gamma _2)\frac{\phi _2}{1+\beta _{21}n_{1a}^*}s_{2a} < 1\) because \(0 \le \gamma \le 1\), \(0 \le \phi \le 1\) and all \(\beta \)’s are positive.
Since \({{\,\textrm{tr}\,}}B_3 > 0\), the first Jury’s condition implies the second ((38) \(\Rightarrow \) (39)). Then, we only have to check the first Jury condition to describe the stability of the system:
We finally have the following condition for the stability of the exclusion equilibrium:
with \(C_2= (1-\gamma _2)\phi _2\) and \(D_2= \frac{\pi _2\gamma _2\phi _2}{1-s_{2a}}\).
This last expression (Eq. 15) gives us an invasion criteria that is larger than 1 if, conversely, species 2 is able to invade the community when rare.
Note that if we remove the competition on one vital rate by setting all the competition coefficients associated to this vital rate to 0, we re-obtain the invasion criteria given by Fujiwara et al. (2011) for their model where a single vital rate was affected by competition. This ensures some internal coherence to the analytical results.
Appendix 2: Coexistence Regions Obtained Through Sensitivity Analysis for the Three Scenarios of Coexistence
To have another, complementary representation of the sensitivities, we estimated coexistence regions for the three parameter sets using the definition given by Barabás et al. (2014b). Note that we arbitrarily use the term region in place of domain to separate the regions found with this sensitivity analysis from the domains of coexistence found through invasion analysis. The method consists in using the sensitivities at the equilibrium for each parameter in order to determine the region of parameter space where species both persists despite changes in parameter values. It is calculated by finding the smallest (positive and negative) perturbation that would lead to the extinction of one of the species from the value of sensitivities and densities at equilibrium, and makes the (crude) approximation that sensitivities are not only valid close to the fixed point but also far away from it. The coexistence regions for the three parameter sets are presented in Fig. 4.
We see here smaller coexistence regions in the third scenario, consistent with this equilibrium being more sensitive (less robust) to perturbations.
Appendix 3: Additional Simulations with S Species, \(S>2\)
The meta-parameter sets, used in the parameter distributions from which the vital rate parameters for each species have been drawn, are provided in Table 3.
Frequencies of outcomes predicted by pairwise invasion criteria—for single-vital-rate-competition models—for a parameter set with 40 species with very close parameters and bimodal distribution of interspecific coefficients drawn to promote situations of opposite exclusion within each pair of species
Histograms of the number of species persisting at equilibrium, with initially 40 species, and for 200 permutations of inter-specific competition coefficients. The bar in gray indicates the number of species persisting at equilibrium for the original \(\alpha _{ij}\) and \(\beta _{ij}\) with \(\text {Corr}(\alpha _{ij}, \beta _{ij})<0\) and reciprocal exclusion for each pair of species
For initially 40 species, a small variance of parameters across species, and opposite competitive hierarchies, we obtained the dynamics illustrated in Fig. 5.
The population dynamics highlighted in Fig. 5 demonstrates that 3000 time steps are enough to reach a stable number of coexisting species.
We verified our hypothesis that a negative correlation between \(\alpha _{ij}\) and \(\beta _{ij}\) leads to opposite hierarchies in sets of coefficients associated to fertility and juvenile survival by plotting the rank of \(\alpha _{ij}\) against the rank of \(\beta _{ij}\). For 40 species, these ranks are plotted in Fig. 6.
The negative correlation between ranks in Fig. 6 confirmed that the negative correlation between \(\alpha _{ij}\) and \(\beta _{ij}\) was creating the expected opposite competitive hierarchies.
However, opposite competitive hierarchies may not be sufficient to promote reciprocal exclusion (e.g., exclusion of 1 by 2 in the fertility competition model and of 2 by 1 in the juvenile survival competition model) of species within each or most pairs of species. To make the comparison between 2 and S-species model, we have to check that we consider situations that are fully comparable, i.e., where species would exclude each other out in a two-species contest on either \(\alpha \) or \(\beta \) competition. We therefore checked if the trade-off between being competitive on \(\alpha \) and \(\beta \) could promote such reciprocal exclusion or priority effects (in models with a single parameter that is density dependent) within pairs of species. We computed the invasion criteria of such simple models (where only one vital rate is affected by competition) for each pair of species composing the communities. For a community of initially 40 species and a small variance in parameters across species, we found the coexistence outcomes in simpler models highlighted in Fig. 7.
We found that for a community of 40 species, the proportions of pairs of species where a priority effect or a reciprocal exclusion are indicated (by the simpler models with density-dependence a single vital rate) are low when we assume only a negative correlation between \(\alpha _{ij}\) and \(\beta _{ij}\).
In fact, when we simply use a negative correlation to create opposite competitive hierarchies, we draw our couple of values (\(\alpha _{ij}\), \(\beta _{ij}\)) in a Gaussian distribution, and then most of the values are close to their means, which does not allow to obtain pairs of species where each species is strongly competitive on a different vital rate, and exclude the other if the competition was on this vital rate only. Therefore, even if there are opposite competitive hierarchies within pairs of species modelled, this setup is unlikely to generate situations where species exclude each other when considering single-vital-rate-competition.
To consider S-species scenarios where species can exclude each other out if competition was solely on \(\alpha \) or solely on \(\beta \) (hereafter referred to as ‘reciprocal exclusion’), we changed our structure of correlation to generate situations where within most pairs of species, each species is strongly competitive on a different vital rate (e.g. species 1 excludes species 2 in the model with only fertility competition and vice-versa in the model with only juvenile survival competition).
Our method to achieve reciprocal exclusion of pairs (for single-vital-rate-competition) consisted in drawing pairs \((a_{ij},b_{ij})\) from a bivariate normal distribution with \(\mu _{a_{ij}}<\mu _{b_{ij}}\) and \(\text {Corr}(a_{ij},b_{ij}) < 0\). We then always assign \((a_{ij},b_{ij})\) to \((\alpha _{ij}, \beta _{ij})\) and \((b_{ji},a_{ji})\) to \((\alpha _{ji}, \beta _{ji})\). In this way, for each pair of species (i, j), species i is competitive on \(\alpha \) and species j is competitive on \(\beta \). We acknowledge that this may be hard to grasp, and suggest to the reader to use the code to create an example.
We computed the invasion criteria for each pair of species and counted how often each invasion scenario in single-vital-rate-competition models, which we represented in the histogram of Fig. 8.
We succeeded to generate reciprocal exclusion within most pairs of species. Moreover, we showed that a permutation of competition coefficients was sufficient to remove the structure of reciprocal exclusion (Fig. 9).
However, as for our simple negative correlation between competitive ranks for \(\alpha \) and \(\beta \), this new parameter set where reciprocal exclusion occurs within most pairs of species does not allow to significantly increase the number of extant species at \(t=3000\) time steps (Fig. 10).
Therefore, even if competition is highly structured in a way that makes species exclude each other out when a single vital rate is density-dependent, which should greatly promote situations of emergent coexistence, we did not observe a positive effect of this structure on species richness in a community larger than \(S=2\). We therefore conclude that emergent coexistence is highly unlikely in many-species contexts.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bardon, G., Barraquand, F. Effects of Stage Structure on Coexistence: Mixed Benefits. Bull Math Biol 85, 33 (2023). https://doi.org/10.1007/s11538-023-01135-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-023-01135-6