Abstract
We consider a minimalist model for the Sterile Insect Technique (SIT), assuming that residual fertility can occur in the sterile male population. Taking into account that we are able to get regular measurements from the biological system along the control duration, such as the size of the wild insect population, we study different control strategies that involve either continuous or periodic impulsive releases. We show that a combination of open-loop control with constant large releases and closed-loop nonlinear control, i.e., when releases are adjusted according to the wild population size estimates, leads to the best strategy in terms of both number of releases and total quantity of sterile males to be released. Last but not least, we show that SIT can be successful only if the residual fertility is less than a threshold value that depends on the wild population biological parameters. However, even for small values, the residual fertility induces the use of such large releases that SIT alone is not always reasonable from a practical point of view and thus requires to be combined with other control tools. We provide applications against a mosquito species, Aedes albopictus, and a fruit fly, Bactrocera dorsalis, and discuss the possibility of using SIT when residual fertility among the sterile males, can occur.


Similar content being viewed by others
References
Anguelov R, Dumont Y, Yatat Djeumen V (2020) Sustainable vector/pest control using the permanent Sterile Insect Technique. Mathematical Methods in the Applied Sciences, pp 1–22. arXiv:1911.02640
Barclay H (2001) Modeling incomplete sterility in a sterile release program: interactions with other factors. Popul Ecol 43:197–206
Bliman PA, Cardona-Salgado D, Dumont Y, Vasilieva O (2019) Implementation of control strategies for sterile insect techniques. Math Biosci 314:43–60
Carey JR (1984) Host-specific demographic studies of the Mediterranean fruit fly Ceratitis capitata. Ecol Entomol 9:261–270
Damiens D, Tjeck PO, Lebon C, Le Goff G, Gouagna LC (2016) The Effects of Age at First Mating and Release Ratios on the Mating Competitiveness of gamma-Sterilised Aedes albopictus males under semi field conditions. Vector Biol J 1:1
Delatte H, Gimonneau G, Triboire A, Fontenille D (2009) Influence of Temperature on Immature Development, Survival, Longevity, fecundity, and gonotrophic cycles of Aedes albopictus, vector of Chikungunya and Dengue in the Indian Ocean. J Med Entomol 46(1):33–41
Dumont Y, Tchuenche JM (2012) Mathematical studies on the sterile insect technique for the Chikungunya disease and Aedes albopictus. J Math Biol 65(5):809–855
Dyck VA, Hendrichs J, Robinson AS (2005) Sterile insect technique: principles and practice in area-wide integrated pest management. Springer, Dordrecht, The Netherlands
Ekesi S, Nderitu P, Rwomushana I (2006) Field infestation, life history and demographic parameters of the fruit fly Bactrocera invadens (Diptera: Tephritidae) in Africa. Bull Entomol Res 96(4):379–386
Iyaloo DP, Oliva C, Facknath S, Bheecarry A (2020) A field cage study of the optimal age for release of radio-sterilized Aedes albopictus mosquitoes in a sterile insect technique program. Entomol Exp Appl 168:137–147
Le Goff G, Damiens D, Ruttee AH, Payet L, Lebon C, Dehecq JS, Gouagna LC (2019) Field evaluation of seasonal trends in relative population sizes and dispersal pattern of Aedes albopictus males in support of the design of a sterile male release strategy. Parasit Vectors 12:81
Manoukis NC, Vargas RI, Carvalho L, Fezza T, Wilson S, Collier T, Shelly TE (2019) A field test on the effectiveness of male annihilation technique against Bactrocera dorsalis (Diptera: Tephritidae) at varying application densities. PLoS ONE 14(3):e0213337
Oliva CF, Jacquet M, Gilles J, Lemperiere G, Maquart P-O, Quilici S (2012) The sterile insect technique for controlling populations of Aedes albopictus (Diptera: Culicidae) on Reunion Island: mating vigour of sterilized males. PLoS ONE 7(11):e49414
Pieterse W, Manrakhan A, Terblanche JS, Addison P (2019) Comparative demography of Bactrocera dorsalis (Hendel) and Ceratitis capitata (Wiedemann) (Diptera: Tephritidae) on deciduous fruit. Bull Entomol Res, pp 1–10
Salum JK, Mwatawala MW, Kusolwa PM, Meyer MD (2014) Demographic parameters of the two main fruit fly (Diptera: Tephritidae) species attacking mango in Central Tanzania. J Appl Entomol 138:441–448
Shelly T, Edu J, McInnis D (2010) Pre-release consumption of methyl eugenol increases the mating competitiveness of sterile males of the oriental fruit fly, Bactrocera dorsalis, in large field enclosures. J Insect Sci 10:8
Strugarek M, Bossin H, Dumont Y (2019) On the use of the sterile insect release technique to reduce or eliminate mosquito populations. Appl Math Modell 68:443–470
Tan K-H, Serit M (1994) Adult population dynamics of Bactrocera dorsalis (Diptera: Tephritidae) in relation to host phenology and weather in two villages of Penang Island, Malaysia. Environ Entomol 23(21):267–275
Tapi M, Bagny-Beilhe L, Dumont Y (2020) Miridae control using sex-pheromone traps. Modeling, analysis and simulations. Nonlinear Anal Real World Appl 54:103082
Vargas RI, Piñero JC, Leblanc L (2015) An overview of pest species of bactrocera fruit flies (Diptera: Tephritidae) and the integration of biopesticides with other biological approaches for their management with a focus on the pacific region. Insects 6(2):297–318
Yusof S, Mohamad Dzomir AZ, Yaakop S (2019) Effect of irradiating Puparia of oriental fruit fly (Diptera: Tephritidae) on adult survival and fecundity for sterile insect technique and quarantine purposes. J Econ Entomol 112(6):2808–2816
Acknowledgements
MSA was supported by the National Council for Scientific and Technological Development (CNPq), by FAPERJ through the “Jovem Cientista do Nosso Estado” Program and by the Getulio Vargas Foundation (FGV, Rio de Janeiro, Brazil) through the “Projeto de Pesquisa Aplicada” Program. YD acknowledges the support of the School of Applied Mathematics of FGV (FGV EMAp) that funded his visit in Rio in 2019. YD is partially supported by the “SIT feasibility project against Aedes albopictus in Reunion Island”, TIS 2B (2020–2021), jointly funded by the French Ministry of Health and the European Regional Development Fund (ERDF). YD is (partially) supported by the DST/NRF SARChI Chair in Mathematical Models and Methods in Biosciences and Bioengineering at the University of Pretoria (Grant 82770). YD is also partially supported by the CeraTIS-Corse project, funded by the call Ecophyto 2019 (Project No: 19.90.402.001), against Ceratitis capitata. This work is done within the framework of the GEMDOTIS project (Ecophyto 2018 funding), that is ongoing in La Réunion. This work was also co-funded by the European Union: Agricultural Fund for Rural Development (EAFRD), by the Conseil Régional de La Réunion, the Conseil Départemental de La Réunion, and by the Centre de Coopération internationale en Recherche Agronomique pour le Développement (CIRAD). We thank the two reviewers for their useful remarks that helped us improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix A: Proof of Theorem 2, p. 13
We consider that we release sterile insects with a period of \(\tau .\) From (22) and (23) we get, for \(t\in [n\tau ,(n+1)\tau ),\)
We impose the condition
This is verified if, for \(t\in [n\tau ,(n+1)\tau ),\)
Since \(\kappa \), introduced in (25), decreases as a function of \(M+F,\) and M and F remain larger than \(M(n\tau )e^{\mu _M (t-n\tau )}\) and \(F(n\tau )e^{\mu _F (t-n\tau )},\) respectively, we get that
Thus, if for \(s\in [0,\tau ),\) it holds
then (M, F) converges asymptotically to 0. This last equation is equivalent to
Since \(\mu _S \ge \mu _M\) and \(\theta \le {{\mathcal {N}}}_F^{-1}\), assuming the additional condition \(\theta \le \dfrac{\mu _S}{\mu _F} {{\mathcal {N}}}_F^{-1}\), one has that all the coefficients and exponents in the r.h.s. of latter expression are positive, so a stronger inequality is obtained if we take \(s=\tau \) for the exponential expressions with positive coefficient and \(s=0\) for the one with negative coefficient. Thus, we impose
This ends the proof.
Appendix B: Proof of Theorem 3, page 12
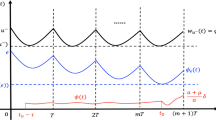
Like in Bliman et al. (2019), we need to adapt the proof of previous Theorem 2, given in Appendix 7. We have, for \(m=0,\dots ,p-1,\) and \(s\in [0,\tau ),\)
We have,
where \(M_{\min }^{np+m}\) and \(F_{\min }^{np+m}\) were introduced in (4). As done above in (29), we impose
By multiplying by \(e^{\mu _S(m\tau +s)}\) both sides of latter inequality, we get
This inequality gives the strongest condition when \(s=\tau \). Thus, we enforce,
We get, for \(m=0,\dots ,p-1\),
The result follows.
Rights and permissions
About this article
Cite this article
Aronna, M.S., Dumont, Y. On Nonlinear Pest/Vector Control via the Sterile Insect Technique: Impact of Residual Fertility. Bull Math Biol 82, 110 (2020). https://doi.org/10.1007/s11538-020-00790-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00790-3