Abstract
We consider a computer simulation, which was found to be faithful to time series data for Caribbean coral reefs, and an analytical model to help understand the dynamics of the simulation. The analytical model is a system of ordinary differential equations (ODE), and the authors claim this model demonstrates the existence of alternative stable states. The existence of an alternative stable state should consider a sudden shift in coral and macroalgae populations, while the grazing rate remains constant. The results of such shifts, however, are often confounded by changes in grazing rate. Although the ODE suggest alternative stable states, the ODE need modification to explicitly account for shifts or discrete events such as hurricanes. The goal of this paper will be to study the simulation dynamics through a simplified analytical representation. We proceed by modifying the original analytical model through incorporating discrete changes into the ODE. We then analyze the resulting dynamics and their bifurcations with respect to changes in grazing rate and hurricane frequency. In particular, a “kick” enabling the ODE to consider impulse events is added. Beyond adding a “kick” we employ the grazing function that is suggested by the simulation. The extended model was fit to the simulation data to support its use and predicts the existence cycles depending nonlinearly on grazing rates and hurricane frequency. These cycles may bring new insights into consideration for reef health, restoration and dynamics.








Similar content being viewed by others
References
Aronson R, Precht W (1997) Stasis, biological disturbance, and community structure of a holocene coral reef. Paleobiology 23(3):326–346
Bak RPM, Carpay MJE, de Ruyter van Steveninck ED (1984) Density of the sea urchin Diadema antillarum before and after mass mortalities on the coral reefs of Curaao. Mar Ecol Prog Ser 17:105–108
Blackwood J, Hastings A, Mumby P (2011) A model-based approach to determine the long-term effects of multiple interacting stressors on coral reefs. Ecol Appl 21(7):2722–2733
Bozec YM, Yakob L, Bejarano S, Mumby PJ (2013) Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos 122:428440
Burnham KP, Anderson DR (2002) Model selection and multimodel inference, 2nd edn. Springer, Fort Collins CO
Graham NA, Bellwood DR, Cinner JE, Hughes TP, Norström AV, Nyström M (2013) Managing resilience to reverse phase shifts in coral reefs. Front Ecol Environ 11:541–548
Heagy J (1992) A physical interpretation of the henon map. Phys D 57:436–446
Hughes TP (1994) Catastrophes, phase shifts, and large-scale degradation of a caribbean coral reef. Science 265(5178):1547–1551
Hughes TP, Reed DC, Boyle MJ (1987) Herbivory on coral reefs: community structure following mass mortalities of sea urchins. J Exp Mar Biol Ecol 113(1):39–59
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Jackson J, Cramer K, Donovan M, Friedlander A, Hooten A, Lam V, Jézéquel JM (2012) Coral reef resilience workshop report. Panama City, Republic of Panama, GCRMN
Knowlton N (1992) Thresholds and multiple stable states in coral reef community dynamics. Am Zool 32(6):674–682
Kusse B, Westwig E (1998) Mathematical physics. Wiley, New York
Martini RM, Krajewski JP, Sazima C, Sazima I (2006) Foraging activity and resource use by three parrotfish species at Fernando de Noronha Archipelago, tropical west atlantic. Mar Biol 149:423–433
McClanahan T, Hendrickn V, Rodrigues M (1999) Varying responses of herbivorous and invertebrate-feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 18:195–203
Mumby PJ (2009) Phase shifts and the stability of macroalgal communities on Caribbean coral reefs. Coral Reefs 28:761–773
Mumby PJ, Hastings A, Edwards HJ (2007) Thresholds and the resilience of caribbean coral reefs. Nature 450(1):98–101
Mumby PJ, Wolff NH, Bozec YM, Chollett I, Halloran P (2014) Operationalizing the resilience of coral reefs in an era of climate change. Conserv Lett 7(3):176–187
Resnik S (2005) A probability path. Birkhauser, Boston
Shulman M, Robertson D (1996) Changes in the coral reefs of San Blas, Caribbean Panama: 1983 to 1990. Coral Reefs 15:231–236
Williams E, Bunkley-Williams L (1990) The world-wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res Bull 355:1–72
Williams ID, Polunin N (2001) Large-scale associations between macroalgal cover and grazer biomass on mid-depth reefs in the Caribbean. Coral Reefs 19:358–366
Williams ID, Polunin NVC, Hendrick VJ (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196
Wood SN (2001) Partially specified ecological models. Ecol Monogr 71(1):1–25
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
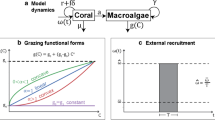
In this paper, our goal was to represent a computer simulation, analytically, which has been shown to be successful at modeling coral reef dynamics. In doing so, our analysis of the simulation has suggested using a grazing term in the form of gM which can be thought of as a Type I functional response with a constant parrot fish population as the predator. Typically, a Type II functional response is thought to be more biologically accurate, so we have included model fitting and bifurcation results for (7) with gM replaced by \(gM/(1+bM)\). This can be thought of as a Type II functional repose with a constant parrot fish population as the predator. Here we add the parameter b which is proportional to the handling time or the times it takes a parrot fish to consume a patch of macroalgae. The model is given in (22) below.
1.1 Type II Response Fits
Here we consider fitting (22) to the simulation data, similar to what was done for (7) in Fig. 4 and Table 3. If we first compare Figs. 4 and 8, we note that except for Trials 8 and 9 there does not appear to be much distinction between the model fits for the simulation data. Comparing Tables 3 and 4, we see a noticeable improvement for the \(R^2\) value for Trials 1, 7 and 9. Also while there are stability issues fitting data for Trial 8 with (7), this problem did not persist with (22); however, the \(R^2\) value is only 0.3.
Stability analysis for Trial 8 fit with Type II functional response for a range of values of grazing rate (g) and time between hurricanes (K). Aside from g and K, all other parameters are from Trial 8 in Table 4. The blue line represents pairs (g, K) for which the cycle is unstable. Pairs above and to the right of the blue line are consistent with stable cycles. No cycles were detected for points below and to the left of the blue line (Color figure online)
1.2 Birfurcation
In Fig. 7, we considered bifurcations about parameter values from Trial 8 with a Type I functional response. If we consider the fits for Trial 8 as in Table 4 with the Type II functional response, then we have the bifurcation diagram as below in Fig. 9. What we notice is that for the parameters chosen the model with the Type II functional response has a much more forgiving bifurcation diagram. In other words, the grazing rate must become very low before hurricane frequency becomes an issue. To see if this is generally the case for Trial 9 we accordingly compared the Type I and Type II functional responses in Figs. 10 and 11. Here, however, we see that the model with the Type I functional response is more forgiving. Overall, the range of dynamics for models with either response function seems to be similar.
Stability analysis for Trial 9 fit with Type I functional response for a range of values of grazing rate (g) and time between hurricanes (K). Aside from g and K, all other parameters are from Trial 9 in Table 4. The blue line represents pairs (g, K) for which the cycle is unstable. Pairs above and to the right of the blue line are consistent with stable cycles. No cycles were detected for points below and to the left of the blue line (Color figure online)
Stability analysis for Trial 9 fit with Type II functional response for a range of values of grazing rate (g) and time between hurricanes (K). Aside from g and K, all other parameters are from Trial 9 in Table 4. The blue line represents pairs (g, K) for which the cycle is unstable. Pairs above and to the right of the blue line are consistent with stable cycles. No cycles were detected for points below and to the left of the blue line (Color figure online)
1.3 Model Selection
The model with the Type II functional response is generally preferred as being derived from first principles and experimentally. Using the Type II response allows a slightly better fit of the trial data compared with using the Type I functional response as noted comparing Tables 3 and 4. This, however, does not imply we should use this model to describe the data. What also needs to be considered here is the principle of parsimony. Biologically, accuracy is extremely important; however, we must be careful about using parameters that the data do not provide enough information to represent. Here the source of data is a simulation where grazing is best represented by a Type I response. If we insist on using the Type II, then our lack of knowledge about the value of the parameter b will likely lead to overfitting the model. Consequently, instead of introducing more biological accuracy, the addition of the parameter b can result in more bias for the system. A good discussion of this point be found on pages 132–134 in Burnham and Anderson (2002).
Rights and permissions
About this article
Cite this article
Ippolito, S., Naudot, V. & Noonburg, E.G. Alternative Stable States, Coral Reefs, and Smooth Dynamics with a Kick. Bull Math Biol 78, 413–435 (2016). https://doi.org/10.1007/s11538-016-0148-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-016-0148-2