Abstract
Background
Chemoimmunotherapy is a standard treatment for advanced non-small-cell lung cancer (NSCLC). However, data on clinical predictive factors remain scarce.
Objective
We aim to identify clinical biomarkers in patients undergoing chemoimmunotherapy.
Methods
This multicenter, real-world cohort study included chemonaive patients who underwent chemoimmunotherapy between December 2018 and May 2022. Multivariate analysis was used to determine associations between survival outcomes and patient background, including baseline neutrophil-to-lymphocyte ratio (NLR) and its dynamic change (ΔNLR). To further investigate the clinical significance of NLR, patients were classified based on their peripheral immune status, defined by a combination of NLR and ΔNLR.
Results
The study included 280 patients with 30.1 months of median follow-up. Multivariate analysis revealed that older individuals, poor performance status, tumor proportion score < 1%, liver metastasis, baseline NLR ≥ 5, and ΔNLR ≥ 0 independently correlated significantly with shorter progression-free and overall survival (OS). Patients with high peripheral immune status (defined as NLR <5 and ΔNLR < 0) significantly improved long-term survival (2-year OS rate of 58.3%), whereas those with low peripheral immune status (defined as NLR ≥ 5 and ΔNLR ≥ 0) had extremely poor outcomes (2-year OS rate of 5.6%). Safety profiles did not differ significantly in terms of severe adverse events and treatment-related death rates despite the patients’ peripheral immune status (P = 0.46 and 0.63, respectively).
Conclusions
Our study provides real-world evidence regarding clinical prognostic factors for the efficacy of chemoimmunotherapy. The combined assessment of baseline NLR and ΔNLR could facilitate the identification of patients who are likely to achieve a durable response from chemoimmunotherapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We hypothesized and demonstrated that chemoimmunotherapy could lead to a dynamic change in the neutrophil-to-lymphocyte ratio (NLR), which could be a novel prognostic factor for patients with advanced non-small cell lung cancer treated with chemoimmunotherapy. |
We classified patients into three groups of peripheral immune status based on their baseline NLR and its dynamic change (ΔNLR). Patients treated with chemoimmunotherapy showed 2-year overall survival rates of 58.3% and 5.6% for patients with high (NLR < 5 and ΔNLR < 0) and low (NLR ≥ 5 and ΔNLR ≥ 0) peripheral immune statuses, respectively. |
Thus, the combination of baseline NLR and ΔNLR may be a key risk stratification factor in patients with advanced non-small cell lung cancer treated with chemoimmunotherapy. |
1 Introduction
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related mortality worldwide [1, 2]. Although molecular targeted therapies for patients with driver mutations are available, improving prognoses in many regions, many patients still lack access to such treatments [3]. For such patients, platinum-based chemotherapy plus immune checkpoint inhibitors (ICIs) are now a standard first-line treatment [4,5,6,7,8,9]. Some patients escape early death and have long-term survival benefits after chemoimmunotherapy.
Various predictive factors for survival are known in patients with NSCLC receiving ICI monotherapy [10]. ICI monotherapy is ineffective for patients with a poor Eastern Cooperative Oncology Group performance status (ECOG-PS) or those requiring oral corticosteroids, proton pump inhibitors (PPIs), or antibiotics before treatment [11]. In addition to clinical features, laboratory parameters, such as the neutrophil-to-lymphocyte ratio (NLR), C-reactive protein, and lactate dehydrogenase, and histological factors, such as programmed death ligand-1 (PD-L1), are predictive biomarkers. Meanwhile, few reports exist regarding predictive factors for patients with NSCLC receiving ICI plus chemotherapy compared with those for patients receiving ICI monotherapy [12,13,14], with these reports having a short follow-up period (median 11.7–18.0 months).
The NLR, defined as the absolute neutrophil count divided by the absolute lymphocyte count, is a promising predictive survival factor in various tumor types [15]. Moreover, the NLR is simple and easy to calculate and can be obtained from routine whole-blood calculations. However, levels of these blood cells are often dynamic rather than static, especially during therapeutic interventions; hence, evaluating NLR dynamic changes is also important.
Recent studies on ICI monotherapy have suggested the special role of early changes in the NLR in predicting the efficacy of immunotherapy for patients with advanced NSCLC [15]. The changes in the NLR before and after immunotherapy can also be easily measured in routine whole-blood tests, as with the baseline NLR. To date, multiple studies have demonstrated that cytotoxic chemotherapy activates and expands lymphocytes via damage-associated molecular patterns in response to tumor cell death while simultaneously decreasing systemic neutrophils [16]. Therefore, we hypothesized that dynamic NLR changes could be a novel prognostic factor of survival for patients with advanced NSCLC treated with chemoimmunotherapy.
2 Material and Methods
2.1 Patient Selection
Consecutive patients with histologically confirmed advanced or recurrent-stage NSCLC were registered through electronic databases of eight institutes in Japan. Patients treated with a first-line combination of pembrolizumab or nivolumab plus ipilimumab with platinum-based chemotherapy were included, while those with positive or untested EGFR gene mutations and ALK rearrangements were excluded according to the Keynote 189 and CheckMate 9LA protocols [4, 6]. Patients whose treatment was initiated between December 2018 and May 2022 were included, and the data collection cutoff date was 30 November 2023.
2.2 Study Design
This multicenter retrospective cohort study collected clinical data from medical records, including age, sex, ECOG-PS, smoking status, histology, driver gene mutations, PD-L1 tumor proportion scores (TPSs), clinical stage, prior thoracic radiotherapy, use of steroids, PPIs, and antibiotics before treatment, brain metastasis, liver metastasis, platinum-based chemotherapy, ICIs, baseline NLR, changes in the NLR before and after treatment (ΔNLR), treatment outcomes, and adverse events (AEs). The PD-L1 TPS in tumor cells was calculated using an anti-PD-L1 antibody (clone 22C3; Dako North America, Inc., CA). Information on medications before treatment included the use of steroids (≥ 10 mg/day of prednisone equivalent) within the 30 days before chemoimmunotherapy initiation, antibiotics within 30 days before treatment initiation, and PPIs at treatment initiation [17,18,19,20,21]. The baseline NLR was defined as the ratio between the numbers of neutrophils and lymphocytes from the peripheral blood count at chemoimmunotherapy initiation. ΔNLR was defined as the difference between the NLR at the start of the second cycle and that at baseline; a high NLR was set as ≥ 5 according to the existing six literature [22], and the ΔNLR cutoff value was zero based on the ease of clinical use and existing literature: ΔNLR ≥ 0 indicates an increase from baseline, and ΔNLR < 0 indicates a decrease [23]. To further evaluate the prognostic relevance of a combination of baseline NLR and ΔNLR, patients were stratified based on the baseline NLR (< 5/≥ 5) and ΔNLR (< 0/≥ 0) into three peripheral immune statuses: high (NLR < 5 and ΔNLR < 0), intermediate (NLR < 5 and ΔNLR ≥ 0 or NLR ≥ 5 and ΔNLR < 0), and low (NLR ≥ 5 and ΔNLR ≥ 0).
Clinical responses were defined according to the Response Evaluation Criteria in Solid Tumors version 1.1 [24]. Progression-free survival (PFS) was defined as the period from the start date of chemoimmunotherapy to the date of disease progression or death from any cause. Overall survival (OS) was determined from the start date of chemoimmunotherapy to the date of death or last follow-up. The safety level was evaluated using the Common Terminology Criteria for Adverse Events version 5.0 based on AE incidence, treatment discontinuation, and treatment-related death (TRD) [25]. Severe AEs (SAEs) were defined as AEs ≥ grade 3.
2.3 Statistical Analyses
Dichotomous variables were compared using the chi-squared or Fisher’s exact test when the smallest expected value was < 5. P values were adjusted using the Bonferroni correction to account for overlap. A list-wise deletion approach was used to handle missing data. Only patients with complete data for all variables of interest were included in the final analysis. Survival was assessed using the Kaplan–Meier method, with the log-rank test for comparison. A landmark time analysis was applied to estimate survival based on changes in NLR. PFS and OS were defined as the period from a 3-week landmark time, including only patients with disease control or those who were alive at 21 days after initiating chemoimmunotherapy for PFS (n = 263) or OS (n = 267), respectively. The median follow-up duration was calculated using only patients who remained alive. To determine the associations between patient characteristics and survival, univariate and multivariate Cox proportional hazards regressions were performed with the variables age, sex, ECOG-PS, smoking status, histology, PD-L1 TPS, cStage, prior thoracic radiotherapy, steroids, PPIs, antibiotics, brain metastasis, liver metastasis, baseline NLR, ΔNLR, platinum-based chemotherapy, and ICIs [10, 11, 17,18,19,20,21,22,23, 26]. Variance inflation factor (VIF) values were calculated to measure the degree of multicollinearity among the explanatory variables. A VIF of > 5 was regarded as a high correlation of the variables [27]. Differences were considered significant at two-sided P values < 0.05. Statistical analyses were performed using R software (version 4.3.0).
3 Results
3.1 Patient Characteristics
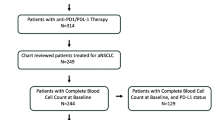
This retrospective study enrolled 321 patients who received chemoimmunotherapy, resulting in 280 patients being included (Supplementary Fig. 1). The patient characteristics are summarized in Table 1. The median patient age was 69.0 years [interquartile range (IQR), 62–73 years]; some older patients (≥ 75 years) were included (18.2%). Most patients were men (79.6%) and smokers (90.0%) and had an ECOG-PS of 0–1 (87.9%). The PD-L1 TPS (high, low, and negative) was balanced. Driver mutations, excluding EGFR and ALK genomic aberrations, were detected in 32 patients (Supplementary Table 1). Some patients had undergone thoracic radiotherapy (10.0%); had taken steroids (6.4%), PPIs (24.6%), or antibiotics (15.4%); and had brain (18.9%) or liver (10.4%) metastases. In most patients, the concomitant ICI was pembrolizumab (92.1%), whereas nivolumab plus ipilimumab was used in some patients. Significant differences were observed in characteristics for ECOG-PS and prior thoracic radiotherapy in both baseline NLR and ΔNLR and for PD-L1 TPS, cStage, history of antibiotics use, and liver metastasis in either baseline NLR or ΔNLR.
3.2 Treatment Effectiveness in All Patients
The median follow-up period was 30.1 (IQR 19.1–43.3) months, with 217 PFS (77.5%) and 169 OS (60.4%) events. The median PFS and OS were 8.0 [95% confidence interval (CI) 6.6–9.3] and 18.5 (95% CI 14.6–22.3) months, respectively (Supplementary Fig. 2).
Multivariate analysis identified age ≥ 75 years [hazard ratio (HR) of 1.54, 95% CI 1.06–2.22, P = 0.022], ECOG-PS of 2–3 (HR of 2.09, 95% CI 1.29–3.39, P = 0.003), PD-L1 TPS < 1% (HR of 1.38, 95% CI 1.01–1.88, P = 0.045), liver metastasis (HR of 1.83, 95% CI 1.14–2.92, P = 0.012), NLR ≥ 5 (HR of 1.64, 95% CI 1.18–2.30, P = 0.003), and ΔNLR ≥ 0 (HR of 1.77, 95% CI 1.26–2.49, P < 0.001) as significant independent predictors of PFS (Fig. 1). Moreover, age ≥ 75 years (HR of 1.90, 95% CI 1.27–2.84, P = 0.002), ECOG-PS of 2–3 (HR of 3.19, 95% CI 1.91–5.32, P < 0.001), PD-L1 TPS < 1% (HR of 1.45, 95% CI 1.01–2.07, P = 0.043), liver metastasis (HR of 2.18, 95% CI 1.34–3.53, P = 0.002), NLR ≥ 5 (HR of 2.16, 95% CI 1.48–3.13, P < 0.001), ΔNLR ≥ 0 (HR of 2.21, 95% CI 1.50–3.28, P < 0.001), and platinum regimen of cisplatin (HR of 0.45, 95% CI 0.22–0.90, P = 0.024) were significant independent OS predictors (Fig. 2). There was no problematic level of multicollinearity among the variables in the PFS and OS multivariate analysis (Supplementary Table 2). Thus, the following six variables showed significant differences between the two classified groups in both PFS and OS: ECOG-PS, PD-L1 TPS, use of antibiotics, liver metastasis, baseline NLR, and ΔNLR (Supplementary Fig. 3).
Univariate and multivariate analyses of progression-free survival in all patients. CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; PPI, proton pump inhibitor; TRT, thoracic radiotherapy
Univariate and multivariate analyses of overall survival in all patients. CI, confidence interval; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; NLR, Neutrophil-to-lymphocyte ratio; PD-L1 TPS, programmed cell death ligand-1 tumor proportion score; PPI, proton pump inhibitor; TRT, thoracic radiotherapy
3.3 Treatment Effectiveness Relative to NLR and ΔNLR
The median PFS and OS for patients with NLR < 5 were 9.9 (95% CI 8.1–12.5) months and 26.6 (95% CI 19.1–36.2) months, respectively, and those for patients with NLR ≥ 5 were 6.5 (95% CI 4.7–8.1) months and 11.6 (95% CI 9.9–16.8) months, respectively (both P < 0.001) (Fig. 3A, B). The objective response rate (ORR) (66.7% versus 54.8%, P = 0.066) and disease control rate (DCR) (91.4% versus 77.4%, P = 0.002) were significantly higher in the low NLR group than in the high NLR group (Supplementary Table 3). Similarly, for patients with ΔNLR < 0, the median PFS and OS were 9.8 (95% CI 7.6–12.1) months and 22.3 (95% CI 18.5–36.2) months, respectively, whereas for patients with ΔNLR ≥ 0 were 6.0 (95% CI 3.9–8.9) months and 13.2 (95% CI 8.7–17.2) months, respectively (P = 0.002 and P < 0.001) (Fig. 3C, D). The ORR (70.3% versus 46.3%, P < 0.001) and DCR (94.3% versus 72.0%, P < 0.001) were significantly higher in the ΔNLR < 0 group than in the ΔNLR ≥ 0 group (Supplementary Table 3).
Kaplan–Meier survival curves with 3-week landmark analysis of patients with advanced NSCLC receiving chemoimmunotherapy grouped by the NLR and ΔNLR. (A) PFS of patients stratified by the baseline NLR. (B) OS of patients stratified by the baseline NLR. (C) PFS of patients stratified by ΔNLR (difference between the NLR at the start of the second cycle and baseline). (D) OS of patients stratified by ΔNLR. (E) PFS of patients stratified by peripheral immune status: high (NLR < 5 and ΔNLR < 0), intermediate (NLR <5 and ΔNLR ≥ 0, or NLR ≥ 5, and ΔNLR < 0), and low (NLR ≥ 5 and ΔNLR ≥ 0). (F) OS of patients stratified by peripheral immune status. CI, confidence interval; NLR, neutrophil-to-lymphocyte ratio; NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival
Patients were classified into peripheral immune groups as follows: high (n = 122), intermediate (n = 133), and low (n = 19) (Supplementary Table 4), with a median PFS of 11.8 (95% CI 9.1–14.3) months, 7.4 (95% CI 6.2–9.8) months, and 1.9 (95% CI: 1.2–6.8) months and a median OS of 31.8 (95% CI 21.7 to not reached) months, 15.1 (95% CI 13.2–20.2) months, and 3.3 (95% CI 2.1–7.9) months, respectively (both P < 0.001). The 2-year PFS rates were 35.3%, 21.6%, and 0%, respectively, and the 2-year OS rates were 58.3%, 37.0%, and 5.6%, respectively (Fig. 3E, F). The ORR (72.1% versus 61.7% versus 15.8%, P < 0.001) and DCR (95.9% versus 87.2% versus 36.8%, P < 0.001) were significantly higher in the high and intermediate peripheral immune groups than in the low immune group (Supplementary Table 3).
3.4 Safety Profiles
Table 2 shows that 95 (34.7%) patients experienced grade ≥ 3 SAEs and 59 (21.5%) discontinued treatment due to SAEs. Ten (3.8%) patients died owing to the following treatment-related AEs: pneumonitis (five patients), febrile neutropenia (three), cardiovascular disorder (one), and bacterial infection (one). Furthermore, the safety profiles did not differ significantly with respect to SAEs, treatment discontinuation, and TRD rates among the peripheral immune statuses (P = 0.463, 0.706, and 0.633, respectively).
4 Discussion
We evaluated the efficacy and safety of chemoimmunotherapy in treatment-naive patients with advanced NSCLC in a real-world setting over a 2-year follow-up period. Combination therapy provided long-term survival for patients with high peripheral immune status (NLR < 5 and ΔNLR < 0) but had extremely poor effectiveness for patients with low peripheral immune status (NLR ≥ 5 and ΔNLR ≥ 0). The safety profiles showed no significant differences in SAEs, treatment discontinuation, and TRD rates despite peripheral immune status. To our knowledge, this is the first study to elucidate clinical outcomes in patients receiving chemoimmunotherapy using the baseline NLR and ΔNLR. Our results can enable early prediction of survival in patients undergoing chemoimmunotherapy.
The survival outcomes in this study are similar to those in clinical trials such as Keynote407 and CheckMate9LA [5, 6]. Moreover, previous real-world reports on chemoimmunotherapy have demonstrated similar survival results [12,13,14]. Consistent with the reported predictive factors for survival in patients with NSCLC treated with chemoimmunotherapy, ECOG-PS, and PD-L1 TPS were identified in this study [12]. We newly identified pretreatment antibiotics, liver metastasis, baseline NLR, and ΔNLR as independent predictive factors for PFS and OS with chemoimmunotherapy, consistent with those reported in previous studies for patients receiving ICI monotherapy [20, 28, 29]. The SAE rate was lower than that reported in previous clinical trials owing to limitations in retrospective clinical data collection from medical records. Therefore, careful attention should be paid to SAE interpretations, discontinuation, and TRD incidences.
Regarding ICI monotherapy, some previous reports have focused on the impact of baseline NLR on prognosis. Tumor cells secrete various chemokines and growth factors, inducing neutrophils to enter the tumors and promote vascular formation. Moreover, neutrophils differentiate into myeloid-derived suppressor cells, thereby preventing T cell activation within the tumor [30, 31]. An immunohistochemical peripheral NLR validation demonstrated that the correlation between the peripheral NLR and clinical outcomes was consistent with the correlation between the intratumoral lymphocyte ratio and the clinical outcomes [32,33,34]. Therefore, a high NLR at baseline implies an increase in the neutrophil count or a decrease in the lymphocyte count within the tumor. Given these findings, the baseline NLR could be a beneficial biomarker in patients treated with chemoimmunotherapy, as reported for ICI monotherapy.
Combining cytotoxic chemotherapy with immunotherapy may enhance antitumor immunity [16]. These are potent endogenous immune adjuvants to the host’s innate immune system, induced through various chemotherapies. Patients who maintained a low NLR during chemotherapy had the most favorable prognosis, whereas those with an increased NLR had the shortest survival [35]. An association between ΔNLR and response to cisplatin-based chemotherapy was found in patients with esophageal and biliary cancers [36, 37], suggesting that ΔNLR may be used as a predictive factor for survival in patients with cancer undergoing chemotherapy, corroborating the basic findings described above. Moreover, the meta-analysis demonstrated that the trends in the NLR were associated with the prognoses of patients receiving ICI monotherapy; notably, a high post-ICI treatment NLR was associated with poorer survival than a high baseline NLR [15], suggesting that ΔNLR can also be an optimal prognostic factor in patients undergoing chemoimmunotherapy. Additionally, the early separation and maintenance of survival curves among the groups based on the composite assessment of the baseline NLR and ΔNLR were most notable in our study. Patients with a high baseline NLR with further increase after the first treatment course experienced markedly poor outcomes. Therefore, combining the NLR and ΔNLR may help identify the subgroup of patients with an unfavorable treatment response.
The incidence of immune-related AEs (irAEs) was suggested to predict enhanced ICI effectiveness [38]. Our study demonstrated that the effectiveness significantly differed among the peripheral immune status (high, intermediate, and low) groups, whereas the SAEs and TRD rates did not significantly differ according to peripheral immune status. In a meta-analysis evaluating the association between the NLR and irAEs, a lower NLR at baseline was significantly associated with higher irAE rates. However, almost all the reports assessed included any-grade irAEs, which may have differed from our results [39]. Thus, the low peripheral immune group (NLR ≥ 5 and ΔNLR ≥ 0) may not benefit from chemoimmunotherapy since its toxicity is comparable with that in the other groups despite its poor effectiveness.
Despite its large multicenter cohort and novel findings, this study had some limitations, including the retrospective nature and the possibility of selection bias. In our cohort, driver gene mutations were not included as an adjustment factor because comprehensive panel testing was not widely available, and detection may have been masked. Moreover, almost all the patients belonged to a single ethnic group (Japanese), which limits the generalizability of these results to other populations. Finally, this study did not compare the efficacy and safety of patients receiving pembrolizumab alone as a control cohort; therefore, further comparative studies are needed.
5 Conclusions
Chemoimmunotherapy provided long-term survival for patients with high peripheral immune status and had extremely poor effectiveness for patients with low peripheral immune status. The safety profiles showed no significant differences in the SAEs, treatment discontinuation, and TRD rates despite the peripheral immune status. Our study demonstrates that combining baseline NLR and ΔNLR can serve as a prognostic factor for survival benefits in patients with advanced NSCLC treated with chemoimmunotherapy. Further prospective investigations are warranted to confirm these results.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. https://doi.org/10.3322/caac.21763.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94. https://doi.org/10.4065/83.5.584.
Kunimasa K, Matsumoto S, Kawamura T, Inoue T, Tamiya M, Kanzaki R, et al. Clinical application of the AMOY 9-in-1 panel to lung cancer patients. Lung Cancer. 2023;179:107190. https://doi.org/10.1016/j.lungcan.2023.107190.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–92. https://doi.org/10.1056/NEJMoa1801005.
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379:2040–51. https://doi.org/10.1056/NEJMoa1810865.
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. https://doi.org/10.1016/S1470-2045(20)30641-0.
Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378:2288–301. https://doi.org/10.1056/NEJMoa1716948.
West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–37. https://doi.org/10.1016/S1470-2045(19)30167-6.
Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III POSEIDON study. J Clin Oncol. 2023;41:1213–27. https://doi.org/10.1200/JCO.22.00975.
Brueckl WM, Ficker JH, Zeitler G. Clinically relevant prognostic and predictive markers for immune-checkpoint-inhibitor (ICI) therapy in non-small cell lung cancer (NSCLC). BMC Cancer. 2020;20:1185. https://doi.org/10.1186/s12885-020-07690-8.
Kawachi H, Yamada T, Tamiya M, Negi Y, Goto Y, Nakao A, et al. Concomitant proton pump inhibitor use with pembrolizumab monotherapy vs immune checkpoint inhibitor plus chemotherapy in patients with Non−Small cell lung cancer. JAMA Netw Open. 2023;6:2322915. https://doi.org/10.1001/jamanetworkopen.2023.22915.
Fujimoto D, Miura S, Yoshimura K, Wakuda K, Oya Y, Haratani K, et al. A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin Res Rep. 2021;3:100265. https://doi.org/10.1016/j.jtocrr.2021.100265.
Banna GL, Cantale O, Muthuramalingam S, Cave J, Comins C, Cortellini A, et al. Efficacy outcomes and prognostic factors from real-world patients with advanced non-small-cell lung cancer treated with first-line chemoimmunotherapy: the Spinnaker retrospective study. Int Immunopharmacol. 2022;110:108985. https://doi.org/10.1016/j.intimp.2022.108985.
Morimoto K, Uchino J, Yokoi T, Kijima T, Goto Y, Nakao A, et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non-small cell lung cancer: a retrospective study. OncoImmunology. 2021;10:1950411. https://doi.org/10.1080/2162402X.2021.1950411.
Guo Y, Xiang D, Wan J, Yang L, Zheng C. Focus on the dynamics of neutrophil-to-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: a meta-analysis and systematic review. Cancers (Basel). 2022;14:5297. https://doi.org/10.3390/cancers14215297.
Sordo-Bahamonde C, Lorenzo-Herrero S, Gonzalez-Rodriguez AP, Martínez-Pérez A, Rodrigo JP, García-Pedrero JM, et al. Chemo-immunotherapy: a new trend in cancer treatment. Cancers (Basel). 2023;15:2912. https://doi.org/10.3390/cancers15112912.
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36:2872–8. https://doi.org/10.1200/JCO.2018.79.0006.
Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, Cabiddu M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers (Basel). 2020;12:546. https://doi.org/10.3390/cancers12030546.
Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline orticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019;37:1927–34. https://doi.org/10.1200/JCO.19.00189.
Pinato DJ, Howlett S, Ottaviani D, Urus H, Patel A, Mineo T, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019;5:1774–8. https://doi.org/10.1001/jamaoncol.2019.2785.
Homicsko K, Richtig G, Tuchmann F, Tsourti Z, Hanahan D, Coukos G, et al. Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann Oncol. 2018;29:X40. https://doi.org/10.1093/annonc/mdy511.001.
Cao D, Xu H, Xu X, Guo T, Ge W. A reliable and feasible way to predict the benefits of Nivolumab in patients with non-small cell lung cancer: a pooled analysis of 14 retrospective studies. OncoImmunology. 2018;7:e1507262. https://doi.org/10.1080/2162402X.2018.1507262.
Chen S, Li R, Zhang Z, Huang Z, Cui P, Jia W, et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Transl Lung Cancer Res. 2021;10:1397–407. https://doi.org/10.21037/tlcr-21-43.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Common Terminology Criteria for Adverse Events (CTCAE). v.5.0. United States Department of Health and Human Services National Institutes of Health—National Cancer Institute. 2017. https://www.meddra.org/. Accessed 31 May 2023.
Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. https://doi.org/10.1016/S1470-2045(17)30380-7.
Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. 2019;72:558–69. https://doi.org/10.4097/kja.19087.
Chen Q, Zhang Z, Li X, Feng S, Liu S. Analysis of prognostic factors affecting immune checkpoint inhibitor therapy in tumor patients exposed to antibiotics. Front Oncol. 2023;13:1204248. https://doi.org/10.3389/fonc.2023.1204248.
Xia H, Zhang W, Zhang Y, Shang X, Liu Y, Wang X. Liver metastases and the efficacy of immune checkpoint inhibitors in advanced lung cancer: a systematic review and meta-analysis. Front Oncol. 2022;12:978069. https://doi.org/10.3389/fonc.2022.978069.
Kalafati L, Mitroulis I, Verginis P, Chavakis T, Kourtzelis I. Neutrophils as orchestrators in tumor development and metastasis formation. Front Oncol. 2020;10:581457. https://doi.org/10.3389/fonc.2020.581457.
Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory interactions between neutrophils, tumor cells and t cells. Front Immunol. 2019;10:1690. https://doi.org/10.3389/fimmu.2019.01690.
Takakura K, Ito Z, Suka M, Kanai T, Matsumoto Y, Odahara S, et al. Comprehensive assessment of the prognosis of pancreatic cancer: peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand J Gastroenterol. 2016;51:610–7. https://doi.org/10.3109/00365521.2015.1121515.
Ohki S, Shibata M, Gonda K, Machida T, Shimura T, Nakamura I, et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol Rep. 2012;28:453–8. https://doi.org/10.3892/or.2012.1812.
Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–81. https://doi.org/10.1038/nri3191.
Liu D, Jin J, Zhang L, Li L, Song J, Li W. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem. 2018;46:1595–605. https://doi.org/10.1159/000489207.
Sato H, Tsubosa Y, Kawano T. Correlation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancer. World J Surg. 2012;36:617–22. https://doi.org/10.1007/s00268-011-1411-1.
Grenader T, Nash S, Plotkin Y, Furuse J, Mizuno N, Okusaka T, et al. Derived neutrophil lymphocyte ratio may predict benefit from cisplatin in the advanced biliary cancer: the ABC-02 and BT-22 studies. Ann Oncol. 2015;26:1910–6. https://doi.org/10.1093/annonc/mdv253.
Wang D, Chen C, Gu Y, Lu W, Zhan P, Liu H, et al. Immune-related adverse events predict the efficacy of immune checkpoint inhibitors in lung cancer patients: a meta-analysis. Front Oncol. 2021;11:631949. https://doi.org/10.3389/fonc.2021.631949.
Zhang W, Tan Y, Li Y, Liu J. Neutrophil to lymphocyte ratio as a predictor for immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Immunol. 2023;14:1234142. https://doi.org/10.3389/fimmu.2023.123414.
Acknowledgements
We thank all patients and staff who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding provided by Osaka University. This work was supported in part by the grant from JSPS KAKENHI (JP23K15187 to T.S.).
Conflict of interest
M.M. reports receiving grants from Ono Pharmaceutical, Merck Sharp and Dohme, Delt-Fly Pharma, and Chugai Pharmaceutical and honoraria for lectures from AstraZeneca, Chugai Pharmaceutical, Nippon Boehringer Ingelheim, Eli Lilly Japan, Novartis, Pfizer, Merck Sharp and Dohme, Bristol-Myers Squibb, Ono Pharmaceutical, Taiho Pharmaceutical, Takeda Pharmaceutical, Nippon Kayaku, Kyowa Kirin, Daiichi-Sankyo, Otsuka Pharmaceutical, and Shionogi Pharmaceutical outside of the submitted work. M.T. reports receiving grants from Ono Pharmaceutical, Japan, Bristol–Myers Squibb, and Boehringer Ingelheim; and honoraria for lectures from Taiho Pharmaceutical, Japan, Eli Lilly, Asahi Kasei Pharmaceutical, Merck Sharp and Dohme, Boehringer Ingelheim, AstraZeneca, Chugai Pharmaceutical, Ono Pharmaceutical, and Bristol-Myers Squibb outside of the submitted work. A.T. reports receiving grants from AstraZeneca, BeiGene, and Daiichi-Sankyo and honoraria for lectures from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Pfizer, Amgen, Kyowa Kirin, Merck BioFarma, Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol–Myers Squibb, Taiho Pharmaceutical, Merck Sharp and Dohme, Takeda Pharmaceutical, Nihon-Kayaku, and Thermo Fischer Scientific outside of the submitted work. The other authors declare no conflicts of interest. K.M., Y.Y., T.S., T.K., Y.K., H.S., S.T., K.U., T.N., I.N., Y.T., and A.K. declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics approval
This cohort study was conducted in accordance with the Declaration of Helsinki and the World Health Organization’s Guidelines for Good Clinical Practice. This study was also approved by the ethical review board of each participating institute.
Consent to participate
An opt-out method was used so that patients and families could refuse to participate in the study.
Consent for publication
Not applicable.
Availability of data and material
Data are available on request from the authors.
Code availability
Not applicable.
Author contributions
K.M.: conceptualization, data curation, formal analysis, investigation, methodology, resources, visualization, writing—original draft, writing—review and editing. Y.Y.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing. T.S.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, visualization, writing—original draft, writing—review and editing. T.K.: conceptualization, data curation, investigation, methodology, resources, visualization, writing—original draft, writing—review and editing. M.M., M.T., Y.K., A.T., H.S., S.T., K.U., and T.N.: investigation, resources, writing—review and editing. I.N.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, visualization, writing—original draft, writing—review and editing. Y.T. and A.K.: project administration, supervision, writing—review and editing. All authors have read and approved the final version of the manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsumoto, K., Yamamoto, Y., Shiroyama, T. et al. Risk Stratification According to Baseline and Early Change in Neutrophil-to-Lymphocyte Ratio in Advanced Non-Small Cell Lung Cancer Treated with Chemoimmunotherapy: A Multicenter Real-World Study. Targ Oncol (2024). https://doi.org/10.1007/s11523-024-01084-7
Accepted:
Published:
DOI: https://doi.org/10.1007/s11523-024-01084-7