Abstract
Background
Little is known regarding the association of cetuximab treatment beyond progression (TBP) with survival among patients with recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC). Although immune checkpoint inhibitors (ICIs) are now considered as first-line treatment, not all patients are suitable for ICIs.
Objective
We conducted a multicenter, retrospective study to evaluate the role of cetuximab TBP in patients with R/M HNSCC after failure of first-line cetuximab-containing chemotherapy.
Patients and Methods
Patients with R/M HNSCC who had tumor progression after first-line cetuximab-containing chemotherapy were included into our study. Oncologic outcomes were estimated including time to cetuximab treatment discontinuation (TTD), progression-free survival 2 (PFS2), overall survival (OS), overall response rate (ORR), and disease control rate (DCR). Multivariate cox regression analysis with survival were conducted. Subgroup analysis with P16 and programmed death ligand 1 expression were performed.
Results
A total of 498 patients were eligible with 259 patients in the TBP group and 239 patients in the non-TBP group. The most common first-line chemotherapy was the EXTREME regimen in both groups. As for second-line treatment, the most common regimen were TPEx in the TBP group and taxane-based chemotherapy in the non-TBP group. Median TTD was 8.7 months in TBP and 5.5 months in non-TBP (p < 0.001). In terms of survival, median OS1 was significant longer in the TBP group than in the non-TBP group [14.1 months versus 10.9 months (p = 0.016)]. Multivariate analysis demonstrated cetuximab TBP was a factor independently associated with OS.
Conclusions
Our retrospective study suggests cetuximab TBP to be effective and to provide better survival for patients with R/M HNSCC after failure of first-line cetuximab-containing chemotherapy. Further prospective studies are warranted to validate the role of cetuximab TBP in R/M HNSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cetuximab treatment beyond progression (TBP) could be an effective treatment for patients with R/M HNSCC after progression on first-line cetuximab-based regimen. |
Median time to cetuximab discontinuation was 8.7 months in TBP and 5.5 months in non-TBP (p < 0.001). |
Median overall survival was 14.1 months in TBP and 10.9 months in non-TBP (p = 0.016). |
1 Introduction
Head and neck squamous cell carcinoma (HNSCC) is the most common malignancy affecting the upper aerodigestive tract [1]. In 2018, there were approximately 880,000 new diagnoses of head and neck cancers in the world, with one-third of these patients occurring in the Asia–Pacific region alone [2]. The prognosis of patients with recurrent or metastatic HNSCC (R/M HNSCC) are poor, with estimated survival of around 8–10 months before 2005 [3]. Given recent treatment advances, the survival of patients with R/M HNSCC has extended to more than 12 months [4]. The EXTREME study demonstrated that cetuximab, a chimeric immunoglobulin G1 monoclonal antibody against epidermal growth factor receptor (EGFR) significantly prolonged survival and improved treatment response [5]. The median overall survival (OS) extended from 7.4 to 10.1 months, median progression-free survival (PFS) from 3.3 to 5.6 months, and overall response rate (ORR) increased from 20 to 36%. Immune checkpoint inhibits (ICI) also play an important role in the treatment of R/M HNSCC. The pivotal phase III Keynote-048 study demonstrated that pembrolizumab monotherapy significantly improved survival in patients with combined positive score (CPS) of 1 or more, while pembrolizumab plus chemotherapy increased survival in the total population [6]. However, there are patients with negative CPS and contraindication for ICIs. Cetuximab-based regimen remains an important first-line treatment for R/M HNSCC. Hence, investigation regarding therapy sequencing after first-line cetuximab-containing regimen remains an interesting point.
Treatment beyond progression (TBP) has been commonly used in anticancer treatment for decades [7]. Several studies have already identified its favorable prognosis to continue molecular-targeted drugs after disease progression, including bevacizumab in metastatic colorectal cancer (mCRC) and trastuzumab in metastatic breast cancer (mBC). Furthermore, cetuximab TBP had also demonstrated survival benefits for patients with mCRC [8]. The CAPRI-GOIM study enrolled 153 patients and found a median PFS of 6.4 versus 4.5 months, for patients with cetuximab TBP and non-TBP, respectively. There was a trend to better overall survival: median 23.7 versus 19.8 months (p = 0.056). Hence, the authors summarized that continuing cetuximab treatment in combination with chemotherapy is of potential therapeutic efficacy in patients with mCRC with all ras wild type. However, little is known regarding the role of cetuximab continuation after progression on first-line cetuximab-containing treatment for R/M HNSCC.
Herein, we conducted a national population-based case-control retrospective study to investigate the oncologic outcome of cetuximab TBP in patients with R/M HNSCC.
2 Patients and Methods
2.1 Patients
The Taiwan Head and Neck Society Cancer Registry Database (THNSCRD) covers over 1500 patients with R/M HNSCC receiving first-line cetuximab-based regimen between 2015 and 2022. This national population-based case-control retrospective study was conducted using the data from THNSCRD, which comprised the information on cancer diagnosis, staging, and treatment. Only such patients who received second-line treatment after progression of first-line cetuximab-containing regimen were included into this study. Some patients continued cetuximab treatment plus other chemotherapy as second-line treatment, while some patients received systemic treatment other than cetuximab as second-line treatment. Thus, patients were classified into the (1) cetuximab TBP group and (2) cetuximab non-TBP group. Patients who had been treated with cetuximab before their recurrent or metastatic disease were excluded. Other exclusion criteria were incomplete second-line treatment, no evaluation of treatment response and irregular follow-up. P16 testing and PD-L1 expression were measured by immunohistochemical stain. Positive PD-L1 expression was defined as tumor proportional score (TPS) > 50%, combined proportional score (CPS) > 1 by Dako 22C3 or tumor cell (TC) PD-L1 > 1% by Dako 28-8. Our study was a retrospective cancer registry analysis and the need for informed consent was waived. This study was approved by the E-Da Hospital Institutional Review Board (EMRP70110N) and was conducted in accordance with the Declaration of Helsinki.
2.2 Statistical Analysis
All clinical variables of these patients were retrospectively collected and presented with frequencies. Patients were classified into the TBP group and no TBP group. Baseline characteristics of each group were compared using chi-square tests. All statistical analysis was performed in SPSS. Time to cetuximab treatment discontinuation (TTD), progression-free survival (PFS), overall survival (OS), overall response rate (ORR), and disease control rate (DCR) were evaluated as oncologic outcomes. TTD was measured as the time from the first day of cetuximab until the last day of cetuximab or final follow-up, while PFS2 was measured as the time from the first day of cetuximab until the day of progression after second-line treatment or final follow-up. OS1 was calculated from the first day of cetuximab until the date of death or final follow-up, while OS2 was calculated from the first day of second-line treatment until the date of death or final follow-up. Objective response criteria were defined as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the RECIST 1.1 guidelines. ORR was defined as CR plus PR, and DCR was defined by CR, PR, plus SD. Kaplan–Meier curves were plotted with TCD, PFS2, OS1, and OS2. Multivariate cox regression analysis with survival were also conducted with “enter” selection to adjust the potential confounders. Subgroup analysis regarding the influences of P16 and PD-L1 were performed. p-Value was two-sided and considered to be significant if p < 0.05.
3 Results
3.1 Patients’ Characteristics
We identified 498 patients with R/M HNSCC between 2015 and 2022 from THNSCRD for outcomes comparison. Patients’ characteristics are presented in Table 1. Among eligible patients, 92% were male, nearly 60% of them younger than 60 years, and more that 80% of them had Eastern Cooperative Oncology Group Performance Status (ECOG PS) 2. Half of our patients had their primary tumor location in the oral cavity, followed by hypopharynx, oropharynx, and larynx. P16 testing was only performed on 30% of our patients with 3–4% positive rate. PD-L1 expression was only estimated on 40% of our patients with 10% positive rate. The main reason of missing PD-L1 data was that archive tissue is not suitable for PD-L1 staining. As for initial stage, 80% of our patients had stage 3–4 disease at diagnosis. Nearly 60% of the patients have ever received curative surgery, induction chemotherapy or chemoradiotherapy. Half of our patients had distant metastasis while the remainder had local recurrent disease. Approximately 55% of our patients had platinum-sensitive disease with platinum-free interval (PFI) of more than 6 months, and 45% of our patients had platinum-refractory disease with PFI of less than 6 months. Patients were stratified according to second-line treatment. In summary, 259 patients continued cetuximab-based regimen as second-line treatment (TBP group), while 239 patients did not continue cetuximab treatment (non-TBP group). Table 1 presents the distributions of characteristics between TBP and non-TBP groups. The clinical variables in these two groups were similar. There were no significant differences in age, gender, ECOG PS, primary tumor location, P16 testing, PD-L1 expression status, initial stage, previous history of treatment, disease status upon enrollment, and PFI.
3.2 Treatment Sequences
Treatment disposition of 498 patients with R/M HNSCC are summarized in Table 2. The chemotherapy regimens were similar between these two groups. The most common first-line chemotherapy regimen was cetuximab, platinum, and 5-fluorouracil (EXTREME regimen), accounting for more than 60% in each group. Moreover, 13% of our patients in each group received cetuximab, platinum, and tegafur/uracil (UPEx regimen). A total of 10% of our patients were treated with cetuximab, platinum, and taxane (TPEx regimen). After progression on first-line chemotherapy, every patient underwent second-line chemotherapy as per our inclusion criteria. For patients in the TBP group, all patients received cetuximab-containing regimen as second-line treatment, accounting for 54% cetuximab plus taxane, 15% cetuximab plus immune checkpoint inhibitors (ICI), 13% EXTREME regimen, and 4% cetuximab plus methotrexate. For patients in the non-TBP group, every patient was treated with non-cetuximab regimen as second-line treatment, accounting for 48% taxane-based regimen, 18% ICI-based regimen, 12% platinum plus 5-fluorouracil, and 6% methotrexate.
3.3 Oncologic Outcomes
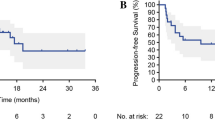
The median follow-up period was 11.1 months. Among these patients, 77% died, and cancer was the main cause of their deaths. Survival curves with TTD, PFS2, and OS 1, 2 are plotted in Fig. 1, respectively. In Fig. 1A, B, TTD and PFS2 were significantly longer in the TBP group than those in the non-TBP group. Median TTD and PFS2 were 7.7 months versus 4.6 months (p < 0.001) and 11.7 months versus 7.7 months (p < 0.001) for TBP and non-TBP group, respectively. In Fig. 1C, D, median OS was significantly better in the TBP group than those in the non-TBP group. Median OS1 and OS2 were 14.1 months versus 10.9 months (p = 0.016) and 9.8 months versus 6.5 months (p = 0.002) for TBP and non-TBP group, respectively. Subgroup analysis suggested survival benefits remained significant regardless PD-L1 expression and P16 status, as shown in Fig. 2. Median OS1 was 22.8 months versus 9.2 months (p = 0.013) and 13.0 months versus 9.9 months (p = 0.010) for patients with PD-L1 positive and PD-L1 negative disease, respectively. Median OS1 was not reached (NR) versus 10.9 months (p = 0.046) and 14.1 months versus 10.9 months (p = 0.002) for patients with P16 positive and P16 negative disease, respectively. Table 3 presents the results of the Cox regression analyses of survival in the total population. After adjusting for potential confounding factors, ECOG PS and cetuximab TBP were independent prognosticators correlated with survival.
4 Discussion
To our best knowledge, this is the first study regarding the prognostic impact of cetuximab TBP in patients with R/M HNSCC. Our study is a retrospective evaluation of data from THNSCRD, and it showed that cetuximab TBP seems to prolong survival for patients with R/M HNSCC after failure of first-line cetuximab-containing treatment. This survival benefits seems to remain significant regardless of PD-L1 expression and P16 status, although these results came from a subgroup analyses. Previous studies have shown that after progression on cetuximab-containing regimen, ICI play important roles in the later-line setting. Keynote-040 compared the efficacy and safety of pembrolizumab versus standard-of-care therapy for the treatment of R/M HNSCC. The results showed clinically meaningful prolongation of OS and favorable safety profile of pembrolizumab, with median OS from 8.4 months with pembrolizumab and 6.9 months with standard of care (p = 0.0161) [9]. Subgroup analysis found that patients with combined proportional score (CPS) ≥ 1 had better prognosis than those with CPS < 1. Checkmate-141, a randomized phase III trial, investigated the role of nivolumab after platinum-based chemotherapy in R/M HNSCC. This study demonstrated that nivolumab resulted in longer OS than treatment with standard therapy [10]. Subgroup analysis from an updated 2-year follow-up report confirmed that patients with PD-L1 ≥ 1% had longer survival than those with PD-L1 < 1 [11]. Although this evidence supports the use of ICI as second-line treatment after progression on a previous cetuximab-based first-line therapy and also the change of first-line treatment option for patients positive for PD-L1 [6], our results suggest that for a subgroup of patients with CPS < 1 or with contraindications for ICIs, cetuximab TBP could be another treatment option for patients with R/M HNSCC. ICIs will be the next line treatment for patients with R/M HNSCC after cetuximab TBP.
TBP is commonly used across several cancer types, including mCRC [12,13,14], mBC [15,16,17,18,19], non-small cell lung cancer (NSCLC) [20], ovarian cancer [21], and gastric cancer [22]. The most employed targeted therapies for TBP are bevacizumab, cetuximab, and panitumumab for mCRC, and trastuzumab for mBC. Bevacizumab is an antiangiogenic used to treat colon cancer, ovarian cancer, brain cancer, and non-small cell lung cancer. The BRiTE study was a large, prospective, observational study that demonstrated that bevacizumab TBP could improve OS for patients with mCRC [12]. Another phase 3 study with ML18147 also confirmed that TBP of bevacizumab plus second-line chemotherapy has clinical benefits in patients with mCRC [15]. Trastuzumab is a humanized monoclonal antibody specific for human epidermal growth factor receptor 2 (HER2) for the treatment of HER2-positive breast cancer. A subgroup analysis from the observational Hermine study suggested that trastuzumab TBP offers a survival benefit to patients with MBC treated with first-line trastuzumab [23]. Moreover, Lim et al. clarified the efficacy of continuation of gefitinib in patients with non-small cell lung cancer (NSCLC) beyond progression [20]. Twelve patients (24.4%) continued gefitinib therapy for 14 months (median value, range 7.2–20.3 months) after progression. The median OS was not reached. Thus, they concluded that it Is beneficial to maintain gefitinib treatment with local treatment such as radiotherapy until symptomatic progression for NSCLC with epidermal growth factor receptor (EGFR) mutation who experience progression on first-line gefitinib treatment.
There are several potential limitations in our study that are inherent to any retrospective study. First, our study only analyzed patients with R/M HNSCC receiving the cetuximab-based regimen as first-line treatment followed by second-line treatment with any regimens. Patients who did not have evaluation for their second-line treatment were excluded. This might be a major bias in this study. Second, this study only investigated the role of cetuximab TBP in the first-line setting, not in later treatment lines. The main reasons were concerns regarding the activity of cetuximab after progression on ICIs and the influence of ICI on subsequent treatment. Thus, patients who received pembrolizumab-based regimen as first-line treatment followed by cetuximab-based regimen as second-line treatment and beyond were excluded. Moreover, low ICI usage in our study was because many patients could not afford the cost of ICIs. Third, this study was a retrospective study with a nonrandomized design. The treatment sequences of these patients were decided at the physician’s discretion, rather than randomly. This may influence the generalizability of our results. Finally, different cetuximab combination regimen might also limit the power of our study.
Our study aims to investigate the prognostic impact of cetuximab TBP in patients with R/M HNSCC. To date, there are no publications regarding prolongation of survival with cetuximab TBP in patients with R/M HNSCC. Appreciating that our retrospective study has several inevitable selection biases, our results provide the first real-world evidence to support the continuation of cetuximab as second-line treatment in patients with R/M HNSCC. Further prospective randomized control studies are warranted to validate our conclusions.
5 Conclusions
Our study investigated the prognostic impact of cetuximab TBP in patients with R/M HNSCC. Based on our results, we show that cetuximab TBP extended survival significantly in patients with R/M HNSCC. Subgroup analysis showed survival benefits remained significant regardless PD-L1 expression and P16 status. In our multivariate analysis, ECOG PS and cetuximab TBP were independent predictors that correlated with survival. Our conclusions are clinically valuable and provide the first real-world evidence to support cetuximab TBP as second-line chemotherapy in patients with R/M HNSCC.
References
Miranda-Filho AFB. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551.
Sung HFJ, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Guigay J, Tahara M, Licitra L, et al. The evolving role of taxanes in combination with cetuximab for the treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: evidence, advantages, and future directions. Front oncol. 2019;9:668.
Magnes T, Wagner S, Kiem D, et al. Prognostic and predictive factors in advanced head and neck squamous cell carcinoma. Int J Mol Sci. 2021;22:4981.
Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–27.
Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:915–1928.
Yerushalmi RKG. Treatment beyond progression: is it moving from belief to evidence? Oncologist. 2010;15:796–8.
Ciardiello F, Normanno N, Martinelli E, et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol. 2016;27:1055–61.
Cohen EEW, Soulières D, Le Tourneau C, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393:156–67.
Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45–51.
Grothey A, Sugrue MM, Purdie DM, et al. Bevacizumab beyond first progression is associated with prolonged overall survival in metastatic colorectal cancer: results from a large observational cohort study (BRiTE). J Clin Oncol. 2008;26:5326–34.
Yamazaki K, Nagase M, Tamagawa H, et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol. 2016;27:1539–46.
Aparicio J, Virgili Manrique AC, Capdevila J, et al. Randomized phase II trial of FOLFIRI-panitumumab compared with FOLFIRI alone in patients with RAS wild-type circulating tumor DNA metastatic colorectal cancer beyond progression to first-line FOLFOX-panitumumab: the BEYOND study (GEMCAD 17–01). Clin Transl Oncol. 2022;24:2155–65.
Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37.
Petrelli F, Barni S. A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer. 2013;13:81–7.
Jackisch C, Welslau M, Schoenegg W, et al. Impact of trastuzumab treatment beyond disease progression for advanced/metastatic breast cancer on survival—results from a prospective, observational study in Germany. Breast. 2014;23:603–8.
Hammerman A, Greenberg-Dotan S, Feldhamer I, et al. Second-line treatment of her2-positive metastatic breast cancer: trastuzumab beyond progression or lapatinib? a population based cohort study. PLoS ONE. 2015;10: e0138229.
von Minckwitz G, Schwedler K, Schmidt M, et al. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3–05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011;47:2273–81.
Lim SW, Park S, Kim Y, et al. Continuation of gefitinib beyond progression in patients with EGFR mutation-positive non-small-cell lung cancer: A phase II single-arm trial. Lung Cancer. 2018;124:293–7.
Pignata S, Lorusso D, Joly F, et al. Carboplatin-based doublet plus bevacizumab beyond progression versus carboplatin-based doublet alone in patients with platinum-sensitive ovarian cancer: a randomised, phase 3 trial. Lancet Oncol. 2021;22:267–76.
Li Q, Jiang H, Li H, et al. Efficacy of trastuzumab beyond progression in HER2 positive advanced gastric cancer: a multicenter prospective observational cohort study. Oncotarget. 2016;7:50656–65.
Extra JM, Antoine EC, Vincent-Salomon A, et al. Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist. 2010;15:799–809.
Acknowledgements
This work was supported by grants from E-Da Cancer Hospital (Grant no.: EDCHP109012).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests
Hung-Ming Wang, Pei-Jen Lou, Muh-Hwa Yang, Tein-Hua Chen, Ming-Yu Lien, Jin-Ching Lin, Jo-Pai Chen, Wei-Chen Lu, Hsueh-Ju Lu, Tai-Lin Huang, Chia-Jui Yen, Shang-Yin Wu, Hui-Ching Wang, and Meng-Che Hsieh declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical approval
This study was approved by the E-Da Hospital Institutional Review Board (EMRP70110N) and was conducted in accordance with the Declaration of Helsinki.
Consent to participate
Written informed consent was waived because this is a retrospective study.
Consent to publish
All authors provided consent for publication.
Data availability
The datasets that support the findings of this study are available from the corresponding (MCH) upon reasonable request.
Code availability
Not applicable.
Authors’ contributions
MCH developed the study design and wrote the manuscript. All authors collected data. All authors critically reviewed and approved the final draft of the manuscript and are accountable for accuracy and integrity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, HM., Lou, PJ., Yang, MH. et al. Cetuximab Treatment beyond Progression in Patients with Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: A Nationwide Population-Based Study (THNS-2021-08). Targ Oncol 19, 51–58 (2024). https://doi.org/10.1007/s11523-023-01028-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01028-7