Abstract
Background
Biliary tract cancers are rare aggressive malignancies typically diagnosed when the disease is metastatic or unresectable, precluding curative treatment.
Objective
We aimed to identify treatment guidelines, real-world treatment patterns, and outcomes for unresectable advanced or metastatic biliary tract cancers in adult patients.
Methods
Databases (MEDLINE, Embase, Cochrane Database of Systematic Reviews) were systematically searched between 1 January, 2000 and 25 November, 2021, and supplemented by hand searches. Eligible records were (1) treatment guidelines and (2) observational studies reporting real-world treatment outcomes, for unresectable advanced or metastatic biliary tract cancers. Only studies performed in the UK, Germany, France, Australia, Canada and South Korea were extracted, to moderate the number of records for synthesis while maintaining representation of a wide range of biliary tract cancer incidences.
Results
A total of 66 relevant unique full-text records were extracted, including 16 treatment guidelines and 50 observational studies. Among guidelines, chemotherapies were most strongly recommended at first line (1L); the combination of gemcitabine and cisplatin (GEMCIS) was recommended as the standard of care in 1L. Recommendations for systemic chemotherapy in the second line (2L) conflicted because of uncertainties around survival benefit. Guidelines on further lines of treatment included a range of locoregional modalities and stenting or best supportive care without providing clear recommendations because of data paucity. Fifty observational studies reporting real-world treatment outcomes were extracted, of which 25 (50%) and 9 (18%) reported outcomes in 1L and 2L, respectively; 22 (44%) reported outcomes for treatments described as ‘palliative’. In 1L, outcomes for systemic chemotherapy were most frequently described (23/25 studies), and GEMCIS was the most common systemic chemotherapy used (10/23 studies) in line with guidelines. Median overall survival with 1L systemic chemotherapy was < 12 months in most studies (16/23; range 4.7–22.3 months). Most 2L studies (10/11) described outcomes for systemic chemotherapy, most commonly for fluoropyrimidine-based regimen (5/10 studies). Median overall survival with 2L systemic chemotherapy was < 12 months in 5/10 studies (range 4.9–21.5 months). Median progression-free survival was reported more rarely than median overall survival. Some studies with small sample sizes or specifically selected patient populations (e.g. higher performance status, or patients who had already responded to treatment) achieved higher median overall survival.
Conclusions
At the time of this review, treatment options for unresectable advanced or metastatic biliary tract cancers confer poor real-world survival. For over a decade, GEMCIS remained the 1L standard of care, highlighting the lack of therapeutic innovation in this indication and the urgent unmet need for novel treatments with improved outcomes in this aggressive condition. Additional observational studies are needed to further understand the effectiveness of currently available treatments, as well as newly available therapies including the addition of immunotherapy in the evolving treatment landscape.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Real-world studies confirmed that most patients with unresectable advanced or metastatic biliary tract cancer only live for under or around 12 months. |
The treatment most commonly used first after diagnosis has remained the same for over 10 years. |
Our research shows that there is an urgent need to find better treatments for patients with unresectable advanced or metastatic biliary tract cancer. |
Since this review was conducted, new treatments have become available, so future real-world studies should be performed to show how well these new treatments work in the real world. |
1 Introduction
Biliary tract cancers (BTCs) comprise a group of rare and aggressive malignancies that originate in the bile duct within or outside the liver (intrahepatic cholangiocarcinoma [iCCA] and extrahepatic cholangiocarcinoma, respectively), in the gallbladder (gallbladder carcinoma) or in the ampulla of Vater [1, 2]. Biliary tract cancer is considered a rare disease and comprises < 1% of all human cancers, but its incidence varies geographically [3] being lower in Europe and higher in the Asia-Pacific and South American regions [4]. Geographic trends in the incidence are associated with regional variations in risk factors, which include parasitic infections, viral hepatitis and bile duct disorders such as primary sclerosing cholangitis [5, 6].
During the early stages of the disease, BTC is generally asymptomatic [7]; this often results in a delayed diagnosis, and up to 80% of patients are diagnosed with unresectable or metastatic disease, which is associated with a significant symptomatic, functional and health-related quality-of-life burden [8,9,10]. Even among patients who are diagnosed at an early disease stage, recurrence after surgical resection, which is the most common curative approach, is highly likely (i.e. up to ~ 80% within 2 years among patients with resected iCCA) [11, 12].
For patients diagnosed with unresectable or metastatic BTC, curative treatments are unavailable and outcomes with available non-curative options are poor; the median overall survival (mOS) with systemic treatment is typically < 12 months [13]. Furthermore the treatment landscape in this indication has seen little innovation in recent years: gemcitabine and cisplatin have remained the standard of care (SoC) in first-line (1L) treatment for over 10 years [14, 15], with other platinum-based combinations and gemcitabine monotherapy as alternative options [16, 17]. For patients who are suitable for additional lines of treatment after progression, at second-line (2L), leucovorin calcium (folinic acid), fluorouracil and oxaliplatin (FOLFOX) are options [2, 13, 16]. In addition, targeted treatments for tested mutations and immune checkpoint inhibitors have been recommended for some patients in the most recent guidelines [2, 16]. Other options include best supportive care and active symptom control, such as biliary drainage or stenting [9, 18].
Given that patients’ fitness and ability to tolerate subsequent treatment deteriorate following progression on 1L treatment, innovation among life-extending treatments at 1L is particularly important and patients have been recommended to join phase III clinical trials in the past [2, 9, 19, 20]. Recently, durvalumab in combination with gemcitabine and cisplatin has received US Food and Drug Administration [21] and European Medicines Agency approval [22] for 1L treatment of unresectable advanced or metastatic BTC based on results from the TOPAZ-1 phase III trial, showing statistically significantly improved overall survival (OS) and progression-free survival (PFS) versus gemcitabine and cisplatin [23]. It is now also recommended by updated National Comprehensive Cancer Network and European Society for Medical Oncology guidelines as 1L treatment in this indication [2, 24]. Other treatments are currently under investigation at 1L, and recently pembrolizumab in combination with gemcitabine and cisplatin showed statistically improved OS versus gemcitabine plus cisplatin in the phase III Keynote-966 trial [25]. Additionally, phase III trials of pemigatinib and futibatinib are ongoing [26, 27].
To our knowledge, no systematic review has broadly explored real-world treatment patterns and outcomes in this indication across treatment modalities, treatment lines and BTC subtypes. Past reviews focussed on specific treatment modalities [28,29,30], treatment lines [9, 31, 32] and BTC subtypes [29] or analysed clinical trial data only [32, 33]. Therefore, this systematised review employed a broad systematic search strategy to identify treatment guidelines as well as real-world treatment patterns and outcomes for unresectable advanced or metastatic BTC, to explore the alignment between recommendations and real-world practice. The systematised approach included a systematic search and screening [34], while extraction and data synthesis focused only on observational studies and guidelines from the UK, Germany, France, South Korea, Australia and Canada, which were selected to maintain representation of a wide range of BTC incidences, aetiologies and treatment practices.
2 Methods
The systematised review followed a systematic search and screening approach as outlined by the Centre for Reviews and Dissemination systematic review guidance. Results are reported according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [35, 36].
2.1 Eligibility Criteria
English language BTC treatment guidelines or observational studies were included if they provided information on the treatment of adult patients diagnosed with unresectable, unresectable advanced or metastatic BTC. Clinical practice guidelines, consensus statements, expert opinions and observational or registry studies with ten or more patients were included. Studies with fewer than ten participants and clinical trials were excluded because of the limited quantitative data available in small studies and the uncertainties associated with findings of small studies and the focus on real-world outcomes, respectively. Further information on study eligibility in terms of the population, intervention(s), comparator(s), outcomes and study (PICOS) design is provided in Table 1.
2.2 Information Sources
Three electronic databases, including MEDLINE, Embase and the Cochrane Database of Systematic Reviews, were searched systematically for records published between 1 January, 2000 and 25 November, 2021 (for search strategies, see Tables S1, S2 and S3 of the Electronic Supplementary Material [ESM]). Additional hand searches of professional society websites, national health technology assessment bodies and policy agencies, and congresses (for 2019–21) were performed to identify additional relevant records and guidelines that are not yet available as full manuscripts or not published in peer-reviewed journals. Furthermore, bibliographies of relevant health technology assessment submissions and systematic reviews were searched to capture any publications not identified in the systematic searches (see Table S4 of the ESM for a full list of additional data sources).
2.3 Study Selection
After an initial deduplication, titles and abstracts of the remaining records were screened by two independent reviewers to identify potentially relevant records. Disagreements were resolved independently by a third reviewer. Full texts of all remaining records were screened in the same manner to identify eligible records for inclusion.
2.4 Data Extraction
Following systematic screening, data extraction from included studies was approached selectively. Only outcomes for the countries of interest, namely the UK, Germany, France, South Korea, Australia and Canada reported as part of multi- or single-country studies were extracted. The countries were selected to maintain representation of a wide range of BTC incidences across geographies. Following this selection process, the majority of reports were found to be from South Korea; to avoid skewing the extracted data overly towards reports from South Korea, reports from South Korea were reduced by prioritising for the most relevant publications (e.g. peer-reviewed publications vs congress publications and prospective vs retrospective studies). Patient characteristics, study setting and design, intervention and comparators, sample sizes and any numerical outcomes were extracted by one researcher and checked for accuracy independently.
2.5 Study Risk of Bias Assessment
Assessments of the risk of bias of observational studies were performed as part of the data extraction using a customised tool based on the Joanna Briggs Institute checklist for cross-sectional studies [37]. Biliary tract cancer treatment guidelines were assessed using a tool adapted from the AGREE II checklist [38].
2.6 Data Synthesis
Extracted records were summarised in a qualitative synthesis. Results were grouped by treatment line, treatment type and outcome, and pooled across BTC subtypes (although subtype-specific data are available in the ESM). Treatment type categories were developed post-hoc to accommodate the wide range of treatments captured in this review (Table S5 of the ESM). These categories were designed to be mutually exclusive to enable meaningful qualitative synthesis; however, studies describing multiple treatments may appear in multiple categories. Treatments were categorised as 1L, 2L, third line (3L) or palliative (where no further details on the line of treatment were provided) based on designation within original reports. Only mOS and mPFS were synthesised and reported here; for other measures of OS and PFS (such as means and landmarks) and other outcomes (such as response rates and adverse events), insufficient data were available to enable a meaningful synthesis. Ranges of mOS and mPFS were created from overall study populations (excluding any subpopulations where larger populations were available) where more than two independent reports provided an outcome. Quantitative analyses or meta-analyses were not performed in this review.
3 Results
3.1 Study Selection
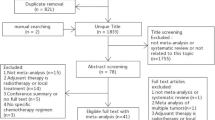
The PRISMA diagram for the selection of eligible records is shown in Fig. 1. Of 2885 initially identified records, 597 were reviewed as full texts and 212 met the inclusion criteria (Table S6 of the ESM). Based on the selection criteria described previously, 53 records were prioritised for extraction; additionally, 13 relevant records from hand searches were identified for extraction, and 66 unique full-text records were extracted. Lists of records excluded during full-text screening and records that met inclusion criteria but were not extracted are provided in Tables S7 and S8 of the ESM.
3.2 Study Characteristics
Table 2 summarises the characteristics of the 66 extracted reports (50 observational studies, 16 guidelines). Nearly all (n = 62) were published in 2011–21. Most reports were from South Korea (n = 20), even though only a prioritised subset of South Korean studies was extracted. Observational studies (n = 50; Table 2) were most commonly retrospective (n = 46; n = 4 prospective reports) and described mixed BTC type cohorts (n = 23) and provided data on 1L and palliative treatments (n = 25 and n = 22, respectively). Patient cohorts most commonly included individuals with mixed cancer stages (n = 37; i.e. including patients with and without metastases). Most studies reported outcomes for systemic chemotherapies (n = 32), including GEMCIS (n = 11), aggregate fluoropyrimidine-based (n = 6) and gemcitabine-based (n = 5) regimen; n = 11 studies only presented outcomes for a mix of systemic chemotherapy regimen without specific details on treatment components or proportions. Demographics of patients included in observational studies broadly matched the patient profile described in the BTC literature (Table S11 of the ESM). In 48 studies reporting age, the median patient age was 64 years (median ages ranged from 54 to 69 years) and men comprised a larger proportion within study cohorts in 37 of 47 studies (median proportion of men: 57%) [39].
Of 16 BTC treatment guidelines (Tables S12 and S13 of the ESM), only four provided recommendations by subtype while others provided general treatment guidelines for BTC. Six guidelines covered general treatment and management, two liver transplantation, one liver disease, and one guideline each focussed on specific treatments or circumstances, including selective internal radiation therapy, proton beam therapy, irreversible electroporation, surgery, care during COVID-19, use of gemcitabine, and interventional or endoscopic care.
Quality assessments are presented in Tables S9 and S10 of the ESM. Observational reports on average only scored 2.0 of 5 possible points; guidelines on average scored 1.6 of 5 points (in both instances, higher scores indicate a lower risk of bias). Assessment results were driven by incomplete descriptions of inclusion criteria (66% of studies) and participants (70%) and a lack of acknowledgement (80%) and mitigation (96%) of potential bias sources. Among the guidelines, most (94%) did not clearly describe relevant patient groups, use systematic methods to identify evidence (87%) or describe methods to formulate guidelines (94%); none clearly described inclusion criteria for selecting evidence.
3.3 Treatment Patterns and Outcomes
3.3.1 First Line
Of the 25 observational studies reporting 1L treatment outcomes, in line with the guidelines most (n = 23) reported on systemic chemotherapy, including GEMCIS (n = 10). However, somewhat in conflict with the guidelines, many other types of systemic chemotherapy were reported at 1L, including mixed systemic chemotherapies (n = 11), GEMOX (n = 1), GEMCARB (n = 1), XP (n = 4) and FOLFIRINOX (n = 1) and aggregates of multiple types of systemic chemotherapy, described as gem- and FP-based regimen (n = 3 and n = 2 respectively) [40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. Notably, for 1L GEMCIS, there is variation in clinical practice regarding limiting treatment to eight cycles or allowing additional cycles [55]. However, of ten reports on 1L GEMCIS, five did not describe the intended duration of treatment with GEMCIS [49,50,51, 57, 58]. Three allowed treatment until progression [52, 56, 65], one limited cisplatin to eight cycles but allowed gemcitabine treatment until progression [53], and one compared eight cycles of GEMCIS to GEMCIS treatment until progression [55]. Although six studies reported treatment exposure data, various units were used that limit comparison across studies; in the three studies that reported a median number of cycles, this ranged from 3.5 to 5 (Table S14 of the ESM) [49, 52, 54].
Among studies reporting on treatments other than systemic chemotherapy, treatment modalities included non-active treatment (best supportive care; n = 2), mixed non-chemotherapy treatments (n = 3; including chemoradiotherapy [CRTx, n = 1] and photodynamic therapy [PDT; n = 2]) and mixed treatments (n = 3; including systemic chemotherapy with radiotherapy [RT] and symptomatic care [n = 1], and systemic chemotherapy with PDT [n = 2]) [43, 48, 56, 62, 63].
Median OS for 1L treatments was provided by 23 reports and was generally poor, being < 12 months in 16 reports (Fig. 2 and Table S11 of the ESM). No evaluated treatments seemed to improve mOS consistently compared with others in this treatment line, as outcomes for each treatment generally exhibited wide overlapping ranges. Where at least three mOS estimates were available across studies for a type of treatment, mOS was 8.4–22.3 months for GEMCIS, 7.4–11.0 months for XP, 7.8–14.6 months for gem-based chemotherapies and 4.7–15.2 for mixed systemic chemotherapies (Fig. 2).
Median overall survival for first-line treatments (A) and median progression-free survival for first-line treatments (B). BTC biliary tract cancer, CCA cholangiocarcinoma, eCCA extrahepatic cholangiocarcinoma, FOLFIRINOX a combination of 5-fluorouracil, leucovorin, oxaliplatin and irinotecan, FP fluoropyrimidine, GBC gallbladder carcinoma, Gem gemcitabine, GEMCARB a combination of gemcitabine and carboplatin, GEMCIS a combination of gemcitabine and cisplatin, GEMOX a combination of gemcitabine and oxaliplatin, iCCA intrahepatic cholangiocarcinoma, mOS median overall survival, mPFS median progression-free survival, XP a combination of capecitabine and cisplatin. Note: only outcomes for overall populations (excluding any outcomes for subpopulations) are provided. Marker sizes are proportional to the number of study participants in each study
Importantly, some studies that achieved high mOS only included small patient groups or selected for patients with a higher performance status or who responded better to treatment. Hyung et al. measured a mOS of 22.3 months for GEMCIS; however, this study only included patients who had not experienced progression after six to eight cycles of GEMCIS and therefore may have responded well to the therapy or were fitter overall than the general BTC population [55]. Median OS for non-systemic chemotherapy-based treatments trended higher compared with the overall sample, although results were only provided in two reports with small patient numbers: Loveday et al. reported 16.4 months with CRTx (n = 18 participants) and Gonzalez-Carmona et al. reported 15 months with PDT (n = 34 participants) [48, 62]. While most reports provided outcomes for mixed BTC populations, available data for BTC subtypes were broadly aligned with ranges generated for mixed BTC populations (Table S15 of the ESM).
Median progression-free survival for 1L treatments was provided by ten reports (Fig. 2B), including GEMCIS (n = 4), GEMCARB (n = 1), GEMOX (n = 1), XP (n = 1), gem- and FP-based chemotherapies (n = 2 and n = 1, respectively), mixed systemic chemotherapies (n = 4) and treatments other than systemic chemotherapy (neoadjuvant chemoradiation ahead of intended liver transplantation; n = 1) [42, 44, 45, 47, 50, 52, 53, 55, 60, 62]. Where at least three mPFS estimates were available across studies for a type of treatment, mPFS was 4.1–12.5 months for GEMCIS and 2.7–9.2 months for mixed systemic chemotherapies. As for mOS, no treatments appeared to consistently improve mPFS over other treatments as outcomes for each treatment generally exhibited wide overlapping ranges (Fig. 2B; Table S11 of the ESM). Where provided, mPFS for subtypes were broadly aligned with mixed BTC populations (Table S16 of the ESM). Studies reporting high mPFS typically co-reported high mOS, and, as described above, generally had small samples or selected specific patient populations. For example, Hyung et al. reported mPFS of 12.5 months for GEMCIS and Loveday et al. reported mPFS of 11.5 months with CRTx [55].
3.3.2 Second and Third Line
Extracted BTC treatment guidelines did not provide clear recommendations on 2L treatment. Unlike at 1L, recommendations for 2L chemotherapies were supported by limited evidence, or were recommended against [66, 67]. Multiple lines of systemic chemotherapy were cautioned against by two guidelines [66, 67], one guideline mentioned the use of FP-based systemic chemotherapy, noting a lack of evidence of benefit [68]. Guidelines further recommended enrolment in clinical trials [3, 66, 69], and the use of targeted therapies in biomarker-defined populations [3, 67] (Table S13 of the ESM).
Eleven reports provided outcomes for 2L treatments [44,45,46,47, 54, 55, 64, 70,71,72,73]. Despite the lack of clear guideline recommendations for chemotherapies at 2L, all reports but one included systemic chemotherapy regimen (n = 10, including mixed regimen n = 7, gem-based n = 2, FP-based n = 5); the other report provided data on GEMOX hepatic arterial infusion (HAI) [73]. No reports provided data on targeted therapies.
Median OS for 2L systemic chemotherapy and GEMOX HAI was provided by ten reports. Consistent with 1L, mOS with 2L chemotherapy was poor (< 12 months in five reports). Where at least three mOS estimates were available across studies for a type of treatment, mOS was 5.2–12.7 months for mixed systemic chemotherapies and 4.9–20.0 months for FP-based chemotherapies (Fig. 3A and Table S11 of the ESM). When compared with other treatments, no treatments appeared to consistently improve mOS as outcomes showed fairly wide ranges within and across treatment types. While GEMOX HAI, gemcitabine and oxaliplatin or capecitabine (categorised as gem-based) and FOLFIRI with bevacizumab treatment (categorised as FP-based) resulted in notably high mOS (20–21.5 months) across three reports, these studies had small participant numbers (n < 15 for all) [45, 72, 73]. The available (limited) subtype-specific data were aligned with those available for mixed samples (Table S13 of the ESM).
Median overall survival for second-line treatments (A) and median progression-free survival for second-line treatments (B). BTC biliary tract cancer, FP fluoropyrimidine, Gem gemcitabine, GEMCARB a combination of gemcitabine and carboplatin, iCCA intrahepatic cholangiocarcinoma, mOS median overall survival, mPFS median progression-free survival. Note: only outcomes for overall populations (excluding any outcomes for subpopulations) are provided. Marker sizes are proportional to the number of study participants in each study
Six reports provided mPFS outcomes for 2L systemic chemotherapy (n = 5 reports) and GEMOX HAI (n = 1; Fig. 3B and Table S11 of the ESM). Systemic chemotherapy regimen included mixed systemic chemotherapies (n = 4; 1.9–4.9 months) and FP-based therapy (n = 2). No treatments appeared to consistently improve mPFS (Fig. 3B). The two studies that described the highest mPFS were two of the three small studies described previously that reported high mOS, for FOLFIRI with bevacizumab treatment and GEMOX HAI [72, 73].
Extracted BTC treatment guidelines did not make recommendations on 3L treatment and the only identified outcome for 3L treatments was in a study subgroup in which patients (n = 5) received mixed chemotherapy (mPFS 7.4 months) [Table S11 of the ESM] [64].
3.3.3 Palliative Treatment
Because the intent of treatment for unresectable advanced or metastatic BTC is not curative, all management of unresectable advanced or metastatic BTC could be considered palliative, including the 1–3L treatments described previously. Guidelines included a range of options as palliative treatments, including various non-chemotherapy-based treatments without a clear designation of a treatment line [66, 67, 69, 74,75,76,77,78,79,80,81,82] as well as treatments termed here as ‘non-active treatments’ such as stenting and biliary drainage [3, 66, 67, 74, 79, 83]. However, BTC treatment guidelines mentioned the paucity of evidence of clear benefit for such treatments and did not make clear recommendations for treatment algorithm and appropriate patient populations.
In line with this, across the extracted observational studies, 22 described the line of treatment as only ‘palliative’, and therefore these reports are discussed separately from studies designating a line of therapy. Of these reports, half included therapies other than systemic chemotherapy (n = 11), eight palliative systemic chemotherapy (without specifying line of therapy, including gem-based chemotherapies [n = 1], GEMCIS [n = 1], mixed systemic chemotherapies [n = 3], mixed systemic chemotherapies as well as non-active treatment [n = 3]) and six mixed treatments [57, 65, 84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102]. In line with BTC treatment guidelines, non-active treatments (including stenting and biliary drainage) were reported in eight studies [57, 85, 86, 88, 93, 100, 102, 103].
Where reported (n = 18), mOS was poor: nine reports showed median survival benefits < 12 months (Fig. 4A and Table S11 of the ESM). Where at least three mOS estimates were available across studies for a type of treatment, mOS was 6.0–38.5 months for treatments other than systemic chemotherapy, 7.8–12.5 months for mixed systemic chemotherapies, 5.6–13.7 months for mixed systemic chemotherapies and non-active treatment, 17.9 months–not reached for mixed treatments and 2.3–8.9 months for non-active treatment. Median OS for the non-chemotherapy treatments PDT/PDT and CTx, ALPPS and RFA were among the highest of all extracted reports (Fig. 4A). However, studies of RFA and PDT/PDT and CTx had small sample sizes (n < 15) and patients receiving RFA and ALPPS were younger (predominantly < 65 years of age) and had comparatively small tumour sizes [89, 94, 97]. Ranges for BTC subtypes were broadly aligned with ranges for mixed BTC samples (Table S15 of the ESM).
Median overall survival for palliative treatments (A) and median progression-free survival for palliative treatments (B). BTC biliary tract cancer, CCA cholangiocarcinoma, eCCA extrahepatic cholangiocarcinoma, GBC gallbladder carcinoma, Gem gemcitabine, GEMCIS a combination of gemcitabine and cisplatin, GEMOX a combination of gemcitabine and oxaliplatin, iCCA intrahepatic cholangiocarcinoma, mOS median overall survival, mPFS median progression-free survival. Note: only outcomes for overall populations (excluding any outcomes for subpopulations) are provided. Marker sizes are proportional to the number of study participants in each study
Only four reports provided mPFS outcomes for palliative treatments, including treatments other than systemic chemotherapy (n = 2), gem-based chemotherapies (n = 1) and GEMCIS (n = 1) [94, 101]. As observed for mOS, a relatively small study (n = 13) of RFA in patients with small tumours (< 3 cm) showed the highest mPFS among identified reports (Fig. 4B, Table S11 of the ESM) [94].
4 Discussion
This systematised review broadly assessed observational treatment patterns and outcomes, unrestricted by treatment type, for unresectable advanced or metastatic BTC. This is a high unmet need indication owing to the aggressive nature of the disease and the high likelihood of detection only at an advanced stage, precluding curative treatment. The review provides a unique perspective across treatment modalities and lines using a systematic search approach; to our knowledge, no other recent work has summarised real-world treatment patterns for this cancer type across treatment modalities.
4.1 Treatment Patterns and Outcomes
For 1L treatment of unresectable advanced or metastatic BTC, clinical practice seems to be generally aligned with treatment guidelines. GEMCIS has been the recommended treatment and SoC for the past 10 years [14, 15], with guidelines suggesting other chemotherapy regimen if an alternative is required. In line with this, the vast majority of 1L studies reported outcomes for systemic chemotherapy, frequently GEMCIS but several other regimen were also evaluated. Furthermore, in line with the general perception that unresectable advanced or metastatic BTC is associated with poor survival outcomes, most studies reported mOS < 12 months for 1L (and later line) treatments, highlighting the need for novel treatments with improved outcomes. Where outcomes for BTC subtypes were available, data were broadly comparable. Notably, mOS and mPFS with GEMCIS in observational studies were broadly aligned with outcomes of the pivotal ABC-02 trial, which established GEMCIS as 1L SoC in this indication [14].
Beyond 1L, guidelines provided relatively weak recommendations because of limited evidence of benefit. At 2L, guidelines highlighted targeted treatments for biomarker defined populations but disagreed on the utility of multiple lines of systemic chemotherapy. Despite all of this, all but one 2L study evaluated systemic chemotherapy. This may indicate that clinicians seek to further treat patients who have progressed on 1L treatment but are fit enough to tolerate further chemotherapy, despite limited evidence of benefit, in recognition of the poor prognosis in this indication. Median OS of 2L treatments ranged from 4.9 to 21.5 months and was comparable across identified treatment options and cancer subtypes. Unexpectedly, the ranges of mOS at 1L and 2L were comparable, but this may be explained by the study selection for patients fit enough to receive further treatment upon progression after 1L. As fewer patients are likely to tolerate additional lines of therapy, this emphasises the importance of maximising the range of effective 1L treatments.
Finally, this review identified substantial evidence for treatments described as ‘palliative’, without specifying the treatment line. The 1L and 2L chemotherapy treatments discussed above can be considered palliative, as the intent of treatment for unresectable advanced or metastatic BTC is not curative, and guidelines also recommended the use of various non-systemic chemotherapy-based treatments in the palliative setting. This is reflected in the range of palliative chemotherapy and non-chemotherapy treatments evaluated in observational studies. Among palliative treatments, mOS ranged from 2.9 months to not reached. This broad range may reflect the palliative treatment subgroup in this review likely includes a mix of treatment lines, possibly including 1L treatment.
4.2 Current Developments in This Indication
As this review only included reports published in or before 2021 from specific countries, guidelines from other countries (e.g. USA National Comprehensive Cancer Network) and guidelines published since 2021 were not included. In the most recent guidelines, durvalumab plus GEMCIS is the recommended 1L treatment, based on the results of the TOPAZ-1 trial in which durvalumab and GEMCIS treatment resulted in significantly improved OS and PFS compared with GEMCIS alone for patients with unresectable advanced or metastatic BTC [2, 23, 24]. Further updates are anticipated following the results of ongoing 1L phase III trials [25,26,27]. Additionally, these guidelines provide stronger 2L treatment recommendations: FOLFOX is considered SoC, and several targeted treatments are recommended for patients with relevant mutations [2, 24]. As the treatment landscape in unresectable BTC continues to evolve, systemic chemotherapies may be altogether replaced by alternative therapy options.
4.3 Areas for Further Research
Data were lacking for some topics explored in this review. We did not identify outcomes for 1L gemcitabine monotherapy (an important treatment option for patients who cannot tolerate 1L GEMCIS), and 2L FOLFOX and 2L targeted treatments, which are important 2L therapies described in most recent guidelines. Additionally, only limited reporting of PFS, response rates and safety data for any treatment modality was identified. In particular, safety data were reported sporadically and inconsistently across the extracted studies, and more granular reporting of treatment toxicities in future studies would provide a better picture of real-world tolerability. Another gap highlighted by this review is treatment effectiveness in BTC subtypes. Nearly half of the studies extracted in this review enrolled single BTC subtypes: the fewest data were available for ampulla of Vater (the rarest BTC subtype) and gallbladder carcinoma, versus iCCA and extrahepatic cholangiocarcinoma. Even when combining results from these studies with available subgroup data from studies enrolling mixed BTC populations, a qualitative synthesis of subgroup data was challenging owing to few commonly reported outcomes across studies even for iCCA and extrahepatic cholangiocarcinoma. Overall, additional observational studies are required to better understand the real-world effectiveness and safety of currently available treatments, and newly available therapies such as durvalumab for BTC.
Last, research focused on the screening and diagnosis of BTC will be fundamental to improving early detection of BTC. This would reduce dependence on palliative treatments for unresectable advanced or metastatic BTC and enable patients to receive curative treatments.
4.4 Strengths and Limitations
This systematised review followed a systematic search and screening approach following Centre for Reviews and Dissemination guidelines and is reported in line with PRISMA guidelines to ensure methodological robustness. A wide variety of relevant databases and an extensive time period were used. In addition, hand searches of additional data sources and congress abstracts published in the last 3 years were conducted, to capture all available reports. However, because of the broad search strategy, this review pragmatically restricted the geographic scope of data extraction to countries that were representative of a wide range of BTC incidences and therefore not all available evidence has been synthesised. Therefore, while this review identified few BTC subtype-specific data, additional data for BTC subtypes may be available from reports that were not extracted because of the restricted scope. A wider review of subtype-specific treatment outcomes may be a subject of future research.
This review did not distinguish outcomes of metastatic and unresectable patients as most reports provided mixed populations. However, cancer stage may affect patient outcomes and therefore introduce variability within and across reports [104].
Data interpretation is further limited by the variability of terminology used in reports and limitations posed by the retrospective study design of most included reports. This affects the description of the BTC stage and resectability, as well as the treatment line and to a lesser extent study endpoints. To mitigate a lack of clear reporting, lines of treatment were assigned by authors based on treatment recommendations in the National Comprehensive Cancer Network and European Society for Medical Oncology guidelines [2, 24].
This review did not perform any in-depth quantitative analysis of data and can therefore only identify trends, but not make definitive conclusions about treatment outcomes. Given the study limitations described above, any quantitative analysis would have considerable uncertainty.
5 Conclusions
Overall, the results of this first broad review of advanced unresectable and metastatic BTC treatment patterns suggest that real-world outcomes in this indication are poor. Across identified treatments, none notably improves patient mOS and mPFS at any treatment line. Furthermore, GEMCIS appears to be a mainstay of 1L treatment, despite its introduction over a decade ago, indicating a lack of innovation in the treatment landscape. This evidence highlights the need for new treatments with improved effectiveness in this aggressive condition. Immunotherapies and targeted treatments, which now are being included in BTC treatment guidelines, represent innovative treatment options in this patient population. However, the effectiveness of these therapies in the real world remains to be assessed in future research.
References
Zhao DY, Lim KH. Current biologics for treatment of biliary tract cancers. J Gastrointest Oncol. 2017;8(3):430–40.
Vogel A, Bridgewater J, Edeline J, Kelley RK, Klümpen HJ, Malka D, et al. Biliary tract cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–40.
Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D, et al. Biliary cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl. 5):v28-37.
Baria K, De Toni EN, Yu B, Jiang Z, Kabadi SM, Malvezzi M. Worldwide incidence and mortality of biliary tract cancer. Gastro Hep Adv. 2022;1(4):618–26.
Bridgewater J, Lopes A, Palmer D, Cunningham D, Anthoney A, Maraveyas A, et al. Quality of life, long-term survivors and long-term outcome from the ABC-02 study. Br J Cancer. 2016;114(9):965–71.
Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016;5(5):61.
Zamani Z, Fatima S. Biliary tract cancer. Treasure Island: StatPearls; 2021.
Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015–27.
Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–38.
Hunter LA, Soares HP. Quality of life and symptom management in advanced biliary tract cancers. Cancers (Basel). 2021;13(20).
Doussot A, Gonen M, Wiggers JK, Groot-Koerkamp B, DeMatteo RP, Fuks D, et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: preoperative and postoperative prognostic models. J Am Coll Surg. 2016;223(3):493-505.e2.
Zhang XF, Beal EW, Bagante F, Chakedis J, Weiss M, Popescu I, et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br J Surg. 2018;105(7):848–56.
Jansen H, Pape UF, Utku N. A review of systemic therapy in biliary tract carcinoma. J Gastrointest Oncol. 2020;11(4):770–89.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81.
Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–74.
Benson AB, D'Angelica MI, Abrams T, Abbott DE, Ahmet A, Anaya DA, et al. Biliary tract cancers, version 1.2023, NCCN clinical practice guidelines in oncology. 2023. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1517. Accessed 21 Apr 2023.
Ghidini M, Pizzo C, Botticelli A, Hahne JC, Passalacqua R, Tomasello G, et al. Biliary tract cancer: current challenges and future prospects. Cancer Manag Res. 2019;11:379–88.
Fornaro L, Vivaldi C, Cereda S, Leone F, Aprile G, Lonardi S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015;34:156.
Vienot A, Neuzillet C. Cholangiocarcinoma: the quest for a second-line systemic treatment. Transl Cancer Res. 2019;8(Suppl. 3):S275–88.
Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer. 2015;121(18):3290–7.
US Food and Drug Administration. FDA D.I.S.C.O. Burst Edition: FDA approval of Imfinzi (durvalumab) for adult patients with locally advanced or metastatic biliary tract cancer. 2022. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-imfinzi-durvalumab-adult-patients-locally-advanced-or#:~:text=On%20September%202%2C%202022%2C%20FDA,or%20metastatic%20biliary%20tract%20cancer. Accessed 21 Apr 2023.
European Medicines Agency. Imfinzi. 2022. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/imfinzi. Accessed 21 Apr 2023.
Oh D-Y, Ruth He A, Qin S, Chen L-T, Okusaka T, Vogel A, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015.
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines®). 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed 6 Dec 2022.
Kelley RK, Ueno M, Yoo C, Finn RS, Furuse J, Ren Z, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–65.
Bekaii-Saab TS, Valle JW, Van Cutsem E, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16(30):2385–99.
Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh D-Y, Moehler MH, et al. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J Clin Oncol. 2020;38(4_Suppl.):TPS600.
Bisello S, Buwenge M, Zamagni A, Deodato F, Macchia G, Arcelli A. Chemoradiation in unresectable biliary tract cancer: a systematic review. Hepatol Pancreat Sci. 2018;02.
Holster JJ, El Hassnaoui M, Franssen S, IJzermans JNM, de Jonge J, Mostert B, et al. Hepatic arterial infusion pump chemotherapy for unresectable intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. Ann Surg Oncol. 2022;29(9):5528–38.
Yan X, Zou H, Lai Y, Ung COL, Hu H. Efficacy and safety of first-line targeted treatment and immunotherapy for patients with biliary tract cancer: a systematic review and meta-analysis. Cancers (Basel), 2022;15(1).
Khankhel ZS, Goring S, Bobiak S, Lamy FX, Nayak D, Garside J, et al. Second-line treatments in advanced biliary tract cancer: systematic literature review of efficacy, effectiveness and safety. Future Oncol. 2022;18(18):2321–38.
Feng L, Wang Y, Xu H, Yi F. Comparison of different first-line systemic therapies in advanced biliary tract cancer based on updated random controlled trials: a systematic review and network meta-analysis. Biomed Res Int. 2022;2022:1720696.
Jiang Y, Zeng Z, Zeng J, Liu C, Qiu J, Li Y, et al. Efficacy and safety of first-line chemotherapies for patients with advanced biliary tract carcinoma: a systematic review and network meta-analysis. Front Oncol. 2021;11: 736113.
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Inf Lib J. 2009;26(2):91–108.
Centre for Reviews and Dissemination. In: Akers J, editor. Systematic reviews: CRD’s guidance for undertaking reviews in health care, 3rd ed. York: Centre for Reviews and Dissemination, University of York; 2013.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–89.
Aromataris E, Munn Z, editors. JBI manual for evidence synthesis. JBI; 2020. https://doi.org/10.46658/JBIMES-20-01.
Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–42.
Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):115–25.
Salati M, Caputo F, Cunningham D, Marcheselli L, Spallanzani A, Rimini M, et al. The A.L.A.N. score identifies prognostic classes in advanced biliary cancer patients receiving first-line chemotherapy. Eur J Cancer. 2019;117:84–90.
Müller L, Mähringer-Kunz A, Jungmann F, Tanyildizi Y, Bartsch F, Czauderna C, et al. Risk stratification in advanced biliary tract cancer: validation of the A.L.A.N. score. J Oncol. 2020;2020.
Kim MJ, Oh DY, Lee SH, Kim DW, Im SA, Kim TY, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer. 2008;8:374.
Ji JH, Kim YS, Park I, Lee SI, Kim RB, Park JO, et al. Chemotherapy versus best supportive care in advanced biliary tract carcinoma: a multi-institutional propensity score matching analysis. Cancer Res Treat. 2018;50(3):791–800.
Fiteni F, Jary M, Monnien F, Nguyen T, Beohou E, Demarchi M, et al. Advanced biliary tract carcinomas: a retrospective multicenter analysis of first and second-line chemotherapy. BMC Gastroenterol. 2014;14:143.
Pracht M, Le Roux G, Sulpice L, Mesbah H, Manfredi S, Audrain O, et al. Chemotherapy for inoperable advanced or metastatic cholangiocarcinoma: retrospective analysis of 78 cases in a single center over four years. Chemotherapy. 2012;58(2):134–41.
Schweitzer N, Kirstein MM, Kratzel AM, Mederacke YS, Fischer M, Manns MP, et al. Second-line chemotherapy in biliary tract cancer: outcome and prognostic factors. Liver Int. 2019;39(5):914–23.
Kang EJ, Choi YJ, Kim JS, Park KH, Oh SC, Seo JH, et al. Prognostic factors for the selection of patients eligible for second-line chemotherapy in advanced biliary tract cancer. Chemotherapy. 2014;60(2):91–8.
Gonzalez-Carmona MA, Bolch M, Jansen C, Vogt A, Sampels M, Mohr RU, et al. Combined photodynamic therapy with systemic chemotherapy for unresectable cholangiocarcinoma. Aliment Pharmacol Ther. 2019;49(4):437–47.
Lee J, Hong TH, Lee IS, You YK, Lee MA. Comparison of the efficacy between gemcitabine-cisplatin and capecitabine-cisplatin combination chemotherapy for advanced biliary tract cancer. Cancer Res Treat. 2015;47(2):259–65.
Brungs D, Aghmesheh M, Sjoquist K, Goldstein D. First-line chemotherapy in advanced biliary tract cancer: an Australian experience. Asia Pac J Clin Oncol. 2015;11:51.
Ha H, Bang JH, Nam AR, Park JE, Jin MH, Bang YJ, et al. Dynamics of soluble programmed death-ligand 1 (sPDL1) during chemotherapy and its prognostic implications in cancer patients: biomarker development in immuno-oncology. Cancer Res Treat. 2019;51(2):832–40.
Park K, Kim KP, Park S, Chang HM. Comparison of gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer. Asia Pac J Clin Oncol. 2017;13(1):13–20.
Kim BJ, Hyung J, Yoo C, Kim KP, Park SJ, Lee SS, et al. Prognostic factors in patients with advanced biliary tract cancer treated with first-line gemcitabine plus cisplatin: retrospective analysis of 740 patients. Cancer Chemother Pharmacol. 2017;80(1):209–15.
Woo SM, Lee WJ, Kim JH, Kim DH, Han SS, Park SJ, et al. Gemcitabine plus cisplatin versus capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer: a retrospective cohort study. Chemotherapy. 2013;59(3):232–8.
Hyung J, Kim B, Yoo C, Kim KP, Jeong JH, Chang HM, et al. Clinical benefit of maintenance therapy for advanced biliary tract cancer patients showing no progression after first-line gemcitabine plus cisplatin. Cancer Res Treat. 2019;51(3):901–9.
Shin DW, Kim MJ, Lee JC, Kim J, Woo SM, Lee WJ, et al. Gemcitabine plus cisplatin chemotherapy prolongs the survival in advanced hilar cholangiocarcinoma: a large multicenter study. Am J Clin Oncol. 2020;43(6):422–7.
Choe JW, Kim HJ, Kim JS. Survival improvement and prognostic factors in recent management of extrahepatic cholangiocarcinoma: a single-center study. Hepatobiliary Pancreat Dis Int. 2020;19(2):153–6.
Beaulieu C, Lui A, Yusuf D, Abdelaziz Z, Randolph B, Batuyong E, et al. A population-based retrospective study of biliary tract cancers in Alberta, Canada. Curr Oncol. 2021;28(1):417–27.
Woo SM, Lee WJ, Han SS, Park SJ, Kim TH, Koh YH, et al. Capecitabine plus cisplatin as first-line chemotherapy for advanced biliary tract cancer: a retrospective single-center study. Chemotherapy. 2012;58(3):225–32.
Cheon J, Lee CK, Sang YB, Choi HJ, Kim MH, Ji JH, et al. Real-world efficacy and safety of nab-paclitaxel plus gemcitabine-cisplatin in patients with advanced biliary tract cancers: a multicenter retrospective analysis. Ther Adv Med Oncol. 2021;13.
Ulusakarya A, Karaboué A, Ciacio O, Pittau G, Haydar M, Biondani P, et al. A retrospective study of patient-tailored FOLFIRINOX as a first-line chemotherapy for patients with advanced biliary tract cancer. BMC Cancer. 2020;20(1):515.
Loveday BPT, Knox JJ, Dawson LA, Metser U, Brade A, Horgan AM, et al. Neoadjuvant hyperfractionated chemoradiation and liver transplantation for unresectable perihilar cholangiocarcinoma in Canada. J Surg Oncol. 2018;117(2):213–9.
Wentrup R, Winkelmann N, Mitroshkin A, Prager M, Voderholzer W, Schachschal G, et al. Photodynamic therapy plus chemotherapy compared with photodynamic therapy alone in hilar nonresectable sholangiocarcinoma. Gut Liver. 2016;10(3):470–5.
Wiazzane N, Chauffert B, Ghiringhelli F. Retrospective analysis of survival benefits of chemotherapy for metastatic or non-resectable intrahepatic cholangiocarcinoma. Clin Res Hepatol Gastroenterol. 2013;37(6):614–8.
You MS, Ryu JK, Choi YH, Choi JH, Huh G, Paik WJH, et al. Therapeutic outcomes and prognostic factors in unresectable gallbladder cancer treated with gemcitabine plus cisplatin. BMC Cancer. 2019;19(1):10.
Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–69.
Barry A, Apisarnthanarax S, O’Kane GM, Sapisochin G, Beecroft R, Salem R, et al. Management of primary hepatic malignancies during the COVID-19 pandemic: recommendations for risk mitigation from a multidisciplinary perspective. Lancet Gastroenterol Hepatol. 2020;5(8):765–75.
Dingle BH, Rumble RB, Brouwers MC. The role of gemcitabine in the treatment of cholangiocarcinoma and gallbladder cancer: a systematic review. Can J Gastroenterol. 2005;19(12):711–6.
Gotfrit J, Goodwin R, Asmis T, Hyde AJ, Alcindor T, Aubin F, et al. Eastern Canadian Gastrointestinal Cancer Consensus Conference 2019. Curr Oncol. 2021;28(3):1988–2006.
Kim BJ, Yoo C, Kim KP, Hyung J, Park SJ, Ryoo BY, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer. 2017;116(5):561–7.
Neuzillet C, Casadei-Gardini A, Brieau B, Vivaldi C, Brandi G, Tougeron D, et al. Fluropyrimidine single agent or doublet chemotherapy as second line treatment in advanced biliary tract cancer. Int J Cancer. 2020;147(11):3177–88.
Guion-Dusserre JF, Lorgis V, Vincent J, Bengrine L, Ghiringhelli F. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21(7):2096–101.
Ghiringhelli F, Lorgis V, Vincent J, Ladoire S, Guiu B. Hepatic arterial infusion of gemcitabine plus oxaliplatin as second-line treatment for locally advanced intrahepatic cholangiocarcinoma: preliminary experience. Chemotherapy. 2013;59(5):354–60.
Alberta Health Services. Cholangiocarcinoma and gallbladder cancer. 2019. Available from: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gi010-biliary.pdf. Accessed 12 Jan 2023.
Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268–9.
Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, et al. Liver transplantation for cholangiocarcinoma and mixed hepatocellular cholangiocarcinoma: Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020;104(6):1125–30.
Burra P, Burroughs A, Graziadei I, Pirenne J, Valdecasas JC, Muiesan O, et al. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–85.
Francoz C, Belghiti J, Castaing D, Chazouillères O, Duclos-Vallée JC, Duvoux C, et al. Model for end stage liver disease exceptions in the context of the French model for end-stage liver disease score-based liver allocation system. Liver Transplant. 2011;17(10):1137–51.
Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, Khor CJ, Ponnudurai R, Moon JH, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28(4):593–607.
Chuong MD, Kaiser A, Khan F, Parikh P, Ben-Josef E, Crane C, et al. Consensus report from the Miami Liver Proton Therapy Conference. Front Oncol. 2019;9:457.
Levillain H, Bagni O, Deroose CM, Dieudonné A, Gnesin S, Grosser OS, et al. International recommendations for personalised selective internal radiation therapy of primary and metastatic liver diseases with yttrium-90 resin microspheres. Eur J Nucl Med Mol Imaging. 2021;48(5):1570–84.
Ruarus AH, Barabasch A, Catalano O, Leen E, Narayanan G, Nilsson A, et al. Irreversible electroporation for hepatic tumors: protocol standardization using the modified Delphi technique. J Vasc Interv Radiol. 2020;31(11):1765-71.e15.
BC Cancer Agency. Upper gastrointestinal cancer (suspected). 2016. Available from: http://www.bccancer.bc.ca/family-oncology-network-site/Documents/UpperGICancer-Part2-PublishCopy-March2017a.pdf. Accessed 12 Jan 2023.
Hwang IG, Song HS, Lee MA, Nam EM, Lim J, Lee KH, et al. Treatment outcomes of gemcitabine alone versus gemcitabine plus platinum for advanced biliary tract cancer: a Korean Cancer Study Group retrospective analysis. Cancer Chemother Pharmacol. 2014;74(6):1291–6.
Huggett MT, Passant H, Hurt C, Pereira SP, Bridgewater JA, Mukherjee S. Outcome and patterns of care in advanced biliary tract carcinoma (ABC): experience from two tertiary institutions in the United Kingdom. Tumori. 2014;100(2):219–24.
Koch C, Franzke C, Bechstein WO, Schnitzbauer AA, Filmann N, Vogl T, et al. Poor prognosis of advanced cholangiocarcinoma: real-world data from a tertiary referral center. Digestion. 2020;101(4):458–65.
Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105(7):839–47.
Mishreki AP, Lim E, Cranefield P, Pascoe S, Jackson S, Stell DA. Low rate of active treatment of patients with hilar cholangiocarcinoma. Ann R Coll Surg Engl. 2013;95(5):349–52.
Höblinger A, Gerhardt T, Gonzalez-Carmona MA, Hüneburg R, Sauerbruch T, Schmitz V. Feasibility and safety of long-term photodynamic therapy (PDT) in the palliative treatment of patients with hilar cholangiocarcinoma. Eur J Med Res. 2011;16(9):391–5.
Hong MJ, Cheon YK, Lee EJ, Lee TY, Shim CS. Long-term outcome of photodynamic therapy with systemic chemotherapy compared to photodynamic therapy alone in patients with advanced hilar cholangiocarcinoma. Gut Liver. 2014;8(3):318–23.
Jegatheeswaran S, Sheen AJ, Siriwardena AK. Contemporary management of perihilar cholangiocarcinoma in a nontransplant hepatopancreatobiliary center. Eur J Gastroenterol Hepatol. 2013;25(9):1099–104.
Kahaleh M, Teoh AY, Siddiqui U, Joshi V, Saunders MD, Saumoy M, et al. Photodynamic therapy in unresectable cholangiocarcinoma: long term international experience of 12 years. Gastrointest Endosc. 2017;85(5):AB608–9.
Lepage C, Cottet V, Chauvenet M, Phelip JM, Bedenne L, Faivre J, et al. Trends in the incidence and management of biliary tract cancer: a French population-based study. J Hepatol. 2011;54(2):306–10.
Kim JH, Won HJ, Shin YM, Kim K-A, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. Am J Roentgenol. 2011;196(2):W205–9.
Saxena A, Bester L, Chua TC, Chu FC, Morris DL. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17(2):484–91.
Beuzit L, Edeline J, Brun V, Ronot M, Guillygomarc’h A, Boudjema K, et al. Comparison of Choi criteria and Response Evaluation Criteria in Solid Tumors (RECIST) for intrahepatic cholangiocarcinoma treated with glass-microspheres Yttrium-90 selective internal radiation therapy (SIRT). Eur J Radiol. 2016;85(8):1445–52.
Li J, Moustafa M, Linecker M, Lurje G, Capobianco I, Baumgart J, et al. ALPPS for locally advanced intrahepatic cholangiocarcinoma: did aggressive surgery lead to the oncological benefit? An international multi-center study. Ann Surg Oncol. 2020;27(5):1372–84.
Vogl TJ, Zangos S, Eichler K, Selby JB, Bauer RW. Palliative hepatic intraarterial chemotherapy (HIC) using a novel combination of gemcitabine and mitomycin C: results in hepatic metastases. Eur Radiol. 2008;18(3):468–76.
Scheuermann U, Kaths JM, Heise M, Pitton MB, Weinmann A, Hoppe-Lotichius M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma: a single-center experience. Eur J Surg Oncol. 2013;39(6):593–600.
Park SY, Kim JH, Yoon HJ, Lee IS, Yoon HK, Kim KP. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin Radiol. 2011;66(4):322–8.
White J, Carolan-Rees G, Dale M, Patrick HE, See TC, Bell JK, et al. Yttrium-90 transarterial radioembolization for chemotherapy-refractory intrahepatic cholangiocarcinoma: a prospective, observational Study. J Vasc Interv Radiol. 2019;30(8):1185–92.
Rostain F, Hamza S, Drouillard A, Faivre J, Bouvier AM, Lepage C. Trends in incidence and management of cancer of the ampulla of Vater. World J Gastroenterol. 2014;20(29):10144–50.
Habermehl D, Haase K, Rieken S, Debus J, Combs SE. Defining the role of palliative radiotherapy in bone metastasis from primary liver cancer: an analysis of survival and treatment efficacy. Tumori. 2011;97(5):609–13.
Peixoto RD, Renouf D, Lim H. A population based analysis of prognostic factors in advanced biliary tract cancer. J Gastrointest Oncol. 2014;5(6):428–32.
Acknowledgements
The authors thank Emma Geldman of Wickenstones Ltd, Abingdon for her contributions to the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca.
Conflict of interest
Michael Paskow, Vivian Peirce, Sukhvinder Johal and Lei Qin are employees of AstraZeneca and receive salaries and stock. Maria Rapoport, Samantha Prince and Ruby Dadzie are employees of Wickenstones Ltd, which has been contracted by AstraZeneca. Wickenstones Ltd was commissioned by AstraZeneca to conduct and publish this research.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
Author contributions
SP conceived the scoping review and designed the review protocol. MR and RD selected eligible records and extracted and interpreted the data. MR performed the narrative synthesis and drafted the report. All authors contributed to the planning of the manuscript, reviewed the work, provided revisions and provided final approval for the publication of this version.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Peirce, V., Paskow, M., Qin, L. et al. A Systematised Literature Review of Real-World Treatment Patterns and Outcomes in Unresectable Advanced or Metastatic Biliary Tract Cancer. Targ Oncol 18, 837–852 (2023). https://doi.org/10.1007/s11523-023-01000-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-023-01000-5