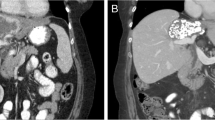
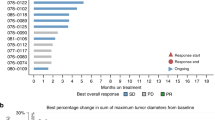
Abstract
Background
Sequencing efforts in patients with cholangiocarcinoma (CCA) have provided insights into molecular mechanisms including fibroblast growth factor receptor (FGFR) alterations. There is a lack of data on outcomes of patients following cessation of FGFR inhibitor (FGFRi) therapy.
Objective
We describe the clinical outcomes following initial FGFRi treatment in CCA harboring FGFR alterations.
Patients and methods
We conducted a multicentric, retrospective analysis of patients with FGFR-altered CCA diagnosed between 2010 and 2021. Median overall survival (OS) and progression-free survival (PFS) analyses were performed using the Kaplan-Meier method.
Results
We identified 88 advanced or metastatic CCA patients, 28 males (31.8%) and 60 females (68.2%), harboring FGFR alterations who received FGFRi. Median PFS on initial FGFRi was 6.6 months (95% confidence interval (CI): 5.5–8.3). Following cessation of first FGFRi therapy, 55% patients received systemic therapy as next line: 67% received chemotherapy or targeted treatment and 33% received another FGFRi. Median PFS for patients who received chemotherapy or targeted agent was 2.1 months (95% CI 1.6–5.7) and for patients who received a second FGFRi was 3.7 months (95% CI 1.5–not evaluable). OS was 2.0 months for patients who did not receive any therapy compared to 8.7 months with chemotherapy and 8.6 months with another FGFRi. In addition, one patient treated with pemigatinib developed FGFR2 M540_I541insMM alteration at time of resistance, which has not been functionally characterized and its effect on protein function remains unknown.
Conclusions
Understanding the mechanisms of resistance with FGFRi is essential to understand sequencing of treatments. In this study, patients received standard chemotherapy in the first line and were fit enough to be considered for subsequent therapy with an FGFRi. Almost half of the patients become ineligible to receive further systemic therapy following progression on FGFRi. As more agents are being introduced, detailed understanding of outcomes following treatment with an FGFRi, including subsequent FGFRi, is essential.



Similar content being viewed by others
References
Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24:4154–61.
Tella SH, Kommalapati A, Borad MJ, Mahipal A. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020;21:e29–41.
Dai S, Zhou Z, Chen Z, Xu G, Chen Y. Fibroblast growth factor receptors (FGFRs): structures and small molecule inhibitors. Cells. 2019;8:614.
Jain A, Borad MJ, Kelley RK, Wang Y, Abdel-Wahab R, Meric-Bernstam F, et al: cholangiocarcinoma With FGFR genetic aberrations: a unique clinical phenotype. JCO Precision Oncol. 2018;2:1–12.
Borad MJ, Gores GJ, Roberts LR. Fibroblast growth factor receptor 2 fusions as a target for treating cholangiocarcinoma. Curr Opin Gastroenterol. 2015;31:264–8.
Javle M, Lowery M, Shroff RT, Weiss KH, Springfeld C, Borad MJ, et al. Phase II study of BGJ398 in patients with FGFR-altered advanced cholangiocarcinoma. J Clin Oncol. 2018;36:276–82.
Papadopoulos KP, El-Rayes BF, Tolcher AW, Patnaik A, Rasco DW, Harvey RD, et al. A Phase 1 study of ARQ 087, an oral pan-FGFR inhibitor in patients with advanced solid tumours. Br J Cancer. 2017;117:1592–9.
Mazzaferro V, El-Rayes BF, Droz Dit Busset M, Cotsoglou C, Harris WP, Damjanov N, et al. Derazantinib (ARQ 087) in advanced or inoperable FGFR2 gene fusion-positive intrahepatic cholangiocarcinoma. Br J Cancer. 2019;120:165–71.
Hoy SM. Pemigatinib: first approval. Drugs. 2020;80:923–9.
Makawita S, Abou-Alfa KG, Roychowdhury S, Sadeghi S, Borbath I, Goyal L, et al. Infigratinib in patients with advanced cholangiocarcinoma with. Future Oncol. 2020;16:2375–84.
Mahipal A, Tella SH, Kommalapati A, Anaya D, Kim R. FGFR2 genomic aberrations: Achilles heel in the management of advanced cholangiocarcinoma. Cancer Treat Rev. 2019;78:1–7.
Abou-Alfa GK, Sahai V, Hollebecque A, Vaccaro G, Melisi D, Al-Rajabi R, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21:671–84.
Mahipal A, Tella SH, Kommalapati A, Yu J, Kim R. Prevention and treatment of FGFR inhibitor-associated toxicities. Crit Rev Oncol Hematol. 2020;155: 103091.
Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81.
Oh D-Y, He AR, Qin S, Chen L-T, Okusaka T, Vogel A, et al: Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022. https://doi.org/10.1056/EVIDoa2200015
Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): a phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021;22:690–701.
Yoo C, Kim K-p, Jeong JH, Kim I, Kang MJ, Cheon J, et al. Liposomal irinotecan plus fluorouracil and leucovorin versus fluorouracil and leucovorin for metastatic biliary tract cancer after progression on gemcitabine plus cisplatin (NIFTY): a multicentre, open-label, randomised, phase 2b study. Lancet Oncol. 2021;22:1560–72.
Bekaii-Saab TS, Valle JW, Cutsem EV, Rimassa L, Furuse J, Ioka T, et al. FIGHT-302: Phase III study of first-line (1L) pemigatinib (PEM) versus gemcitabine (GEM) plus cisplatin (CIS) for cholangiocarcinoma (CCA) with FGFR2 fusions or rearrangements. J Clin Oncol. 2020;38:TPS592.
Borad MJ, Bridgewater JA, Morizane C, Shroff RT, Oh D-Y, Moehler MH, et al. A phase III study of futibatinib (TAS-120) versus gemcitabine-cisplatin (gem-cis) chemotherapy as first-line (1L) treatment for patients (pts) with advanced (adv) cholangiocarcinoma (CCA) harboring fibroblast growth factor receptor 2 (FGFR2) gene rearrangements (FOENIX-CCA3). J Clin Oncol. 2020;38:TPS600.
Borad MJ, Champion MD, Egan JB, Liang WS, Fonseca R, Bryce AH, et al. Integrated genomic characterization reveals novel, therapeutically relevant drug targets in FGFR and EGFR pathways in sporadic intrahepatic cholangiocarcinoma. PLoS Genet. 2014;10: e1004135.
Graham RP, Barr Fritcher EG, Pestova E, Schulz J, Sitailo LA, Vasmatzis G, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:1630–8.
Wang J, Xing X, Li Q, Zhang G, Wang T, Pan H, et al. Targeting the FGFR signaling pathway in cholangiocarcinoma: promise or delusion? Ther Adv Med Oncol. 2020;12:1758835920940948.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Infigratinib approval letter, May 28, 2001. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma.
U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Pemigatinib approval letter, April 20, 2020. https://cacmap.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion.
Krook MA, Lenyo A, Wilberding M, Barker H, Dantuono M, Bailey KM, et al. Efficacy of FGFR inhibitors and combination therapies for acquired resistance in FGFR2-fusion cholangiocarcinoma. Mol Cancer Ther. 2020;19:847–57.
Goyal L, Saha SK, Liu LY, Siravegna G, Leshchiner I, Ahronian LG, et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2017;7:252–63.
Silverman IM, Hollebecque A, Friboulet L, Owens S, Newton RC, Zhen H, et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11:326–39.
Goyal L, Shi L, Liu LY, Fece de la Cruz F, Lennerz JK, Raghavan S, et al. TAS-120 overcomes resistance to ATP-competitive fgfr inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma. Cancer Discov. 2019;9:1064–79.
Varghese AM, Patel J, Janjigian YY, Meng F, Selcuklu SD, Iyer G, et al: Noninvasive detection of polyclonal acquired resistance to FGFR inhibition in patients with cholangiocarcinoma harboring FGFR2 alterations. 2021. 44-50.
Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17:557–88.
Cleary JM, Iyer G, Oh D-Y, Mellinghoff IK, Goyal L, Ng MCH, et al. Final results from the phase I study expansion cohort of the selective FGFR inhibitor Debio 1,347 in patients with solid tumors harboring an FGFR gene fusion. J CLin Oncol. 2020;38:3603–3603.
Javle MM, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Waldschmidt DT, et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J Clin Oncol. 2021;39:265–265.
Goyal L, Meric-Bernstam F, Hollebecque A, Valle JW, Morizane C, Karasic TB, et al. FOENIX-CCA2: A phase II, open-label, multicenter study of futibatinib in patients (pts) with intrahepatic cholangiocarcinoma (iCCA) harboring FGFR2 gene fusions or other rearrangements. J Clin Oncol. 2020;38:108–108.
Meric-Bernstam F, Bahleda R, Hierro C, Sanson M, Bridgewater J, Arkenau H-T, et al. Futibatinib, an irreversible FGFR1–4 inhibitor, in patients with advanced solid tumors harboring FGF/FGFR Aberrations: a phase I dose-expansion study. Cancer Discov. 2022;12:402–15.
Oh D-Y, Lee K-H, Lee D-W, Kim TY, Bang J-H, Nam A-R, et al. Phase II study assessing tolerability, efficacy, and biomarkers for durvalumab (D) ± tremelimumab (T) and gemcitabine/cisplatin (GemCis) in chemo-naïve advanced biliary tract cancer (aBTC). J Clin Oncol. 2020;38:4520–4520.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was partially supported by the National Institutes of Health Grant P30CA15083 (Mayo Clinic Comprehensive Cancer Center grant).
Conflicts of interest/Competing interests
Jennifer J. Gile, Vanessa Wookey, Tyler J. Zemla, Qian Shi, Zhaohui Jin, Steven R. Alberts, Robert R. McWilliams, Wen Wee Ma, Mitesh Borad, Tanios S. Bekaii-Saab, Nguyen H. Tran, and Amit Mahipal declare that they have no conflicts of interest that might be relevant to the contents of this article.
Ethics approval
The study was reviewed and approved by the Mayo Clinic Institutional Review Board and deemed not to require informed consent or consent for publication.
Consent to participate
Not required.
Consent for publication
Not required.
Availability of data
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Authors’ contributions
JG: study conception and design, data collection, analysis, manuscript preparation. VW: study conception and design, data collection, analysis, manuscript preparation. TZ: data collection, analysis, manuscript preparation. QS: data collection, analysis, manuscript preparation. ZJ: study conception and design, analysis, manuscript preparation. SA: study conception and design, analysis, manuscript preparation. RM: study conception and design, analysis, manuscript preparation. WWM: study conception and design, analysis, manuscript preparation. MB: study conception and design, analysis, manuscript preparation. TB-S: study conception and design, analysis, manuscript preparation. NT: study conception and design, data collection, analysis, manuscript preparation. AM: study conception and design, data collection, analysis, manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gile, J.J., Wookey, V., Zemla, T.J. et al. Outcomes following FGFR Inhibitor Therapy in Patients with Cholangiocarcinoma. Targ Oncol 17, 529–538 (2022). https://doi.org/10.1007/s11523-022-00914-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-022-00914-w