Abstract
Background
Sorafenib represents one of the therapeutic strongholds for advanced hepatocellular carcinoma (HCC), but unfortunately, predictive factors are lacking. We previously reported that the VEGF single nucleotide polymorphisms (SNPs) rs2010963 and rs4604006 might correlate with clinical outcomes in sorafenib-treated HCC patients.
Objective
The objective of the ALICE-2 study is to define a prognostic angiogenesis profile to better identify HCC patients who are more likely to benefit from sorafenib treatment.
Patients and methods
From 2008 to 2015, all consecutive HCC patients receiving sorafenib according to the Italian label were tested for specific HIF-1α, VEGF, and VEGFR SNPs. Results from angiogenesis genotyping were then correlated with clinical outcome parameters.
Results
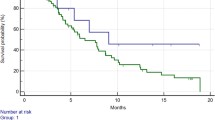
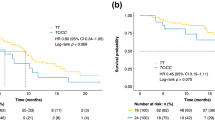
Globally, a total of 210 patients were enrolled. At multivariate analysis rs12434438 of HIF1α, rs2010963 of VEGF-A, and rs4604006 of VEGF-C were confirmed as independent predictive factors. At the combined analysis of significant SNPs, the presence of two favourable alleles of rs2010963 and rs4604006 of VEGF compared to only one or to none favourable alleles, was able to identify three separate patients populations with different time-to-progression (TTP) (10.8 vs. 5.6 vs. 3.7 months, respectively; p < 0.0001) and overall survival (OS) (19.0 vs. 13.5 vs. 7.5 months, respectively; p < 0.0001). Furthermore, the presence of the GG genotype of rs12434438 (HIF-1α) seemed able to select a population with a particularly poor outcome, independently from the clinical effect of the two VEGF SNPs (TTP: 2.6 months, HR: 0.54, p = 0.0374; OS: 6.6 months, p = 0.0061, HR: 0.43).
Conclusions
Our findings show that polymorphism analysis of HIF-1α, VEGF, and VEGFR genes may represent a prognostic panel to better identify HCC patients who are more likely to benefit from sorafenib treatment.



Similar content being viewed by others
Change history
10 November 2020
The listing of the author names and affiliations, which previously read.
References
Faloppi L, Scartozzi M, Maccaroni E, Paolo MDP, Berardi R, Del Prete M, et al. Evolving strategies for the treatment of hepatocellular carcinoma: from clinical-guided to molecularly-tailored therapeutic options. Cancer Treat Rev. 2011;37(3):169–77.
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomized, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Galmiche A, Chauffert B, Barbare JC. New biological perspectives for the improvement of the efficacy of sorafenib in hepatocellular carcinoma. Cancer Lett. 2014;346:159–62.
Holderfield MM, Nagel TE, Stuart DD. Mechanism and consequences of RAF kinase activation by small-molecule inhibitors. Br J Cancer. 2014;111(4):640–5.
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. A Randomised Phase 3 trial of lenvatinib vs. sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma. Lancet. 2018;391:1163–73.
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66.
Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63.
Zhu AX, Kang YK, Yen CJ, Finn RS, Galle PR, Llovet JM, et al. REACH-2: a randomized, double-blind, placebo-controlled phase 3 study of ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha-fetoprotein (AFP) following first-line sorafenib. J Clin Oncol. 2018;36:4003.
Bristol-Myers Squibb Announces Results from CheckMate -459 study evaluating opdivo (nivolumab) as a First-Line Treatment for Patients with Unresectable Hepatocellular Carcinoma. Bristol-Myers Squibb. 2019. https://bit.ly/2x70MSX. Accessed 24 Jun 2019.
Sangro B, Park JW, Dela Cruz CM, Anderson J, Lang L, Neelyet J, et al. A randomized, multicenter, Phase III study of nivolumab vs. sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol. 2016;34(Suppl. 15):TPS4147.
Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HJ, et al. Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs. best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2019;37(Suppl. 15):4004.
Casadei Gardini A, Foca F, Scartozzi M, Silvestris N, Tamburini E, Faloppi L, et al. Metronomic capecitabine versus best supportive care as second-line treatment in hepatocellular carcinoma: a retrospective study. Sci Rep. 2017;7:42499.
Sangro B, Gomez-Martin C, de la Mata M, Iñarrairaegui M, Garralda E, Barrera P, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol. 2013;59:81–8.
Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–51.
El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502.
Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52.
Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, et al. Ten years of sorafenib in hepatocellular carcinoma: are there any predictive and/or prognostic markers? World J Gastroenterol. 2018;24(36):4152–63.
Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76.
Peng S, Wang Y, Peng H, Chen D, Shen S, Peng B, et al. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology. 2014;60(4):1264–77.
Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992;12:5447–54.
Jiang B, Rue E, Wang GL, Roe R. Semenza GL Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor-1. J Biol Chem. 1997;30:17771–8.
Wang W, Xu GL, Jia WD, Wang ZH, Li JS, et al. Expression and correlation of hypoxia-inducible factor-1a, vascular endothelial growth factor and microvessel density in experimental rat hepatocarcinogenesis. J Int Med Res. 2009;2:417–25.
Scartozzi M, Faloppi L, Svegliati Baroni S, Loretelli C, Piscaglia F, Iavarone M, et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: the ALICE-1 study. Int J Cancer. 2014;135:1247–56.
Formento JL, Etienne-Grimaldi MC, Francoual M, Pagès G, Onesto C, Formento P, et al. Influence of the VEGF-A 936C > T germinal polymorphism on tumoral VEGF expression in head and neck cancer. Pharmacogenomics. 2009;10:1277–83.
Chen MH, Tzeng CH, Chen PM, Lin JK, Lin TC, Chen WS, et al. VEGF-406T → polymorphism and its association with VEGF expression and outcome to FOLFOX-4 treatment in patients with colorectal carcinoma. Pharmacogenomics J. 2011;11(3):227–36.
North Carolina State University. 2019. www.statgen.ncsu.edu/powermarker.
Pages G, Puyssegur J. Transcriptional regulation of the vascular endothelial growth factor gene—a concert of activating factors. Cardiovasc Res. 2005;65:564–73.
Ruggiero D, Dalmasso C, Nutile T, Sorice R, Dionisi L, Aversano M, et al. Genetics of VEGF serum variation in human isolated populations of cilento: importance of VEGF polymorphisms. PLoS One. 2011;6(2):e16982.
Al-Habboubi HH, Mahdi N, Abu-Hijleh TM, Abu-Hijleh FM, Sater MS, Almawi WY. The relation of vascular endothelial growth factor (VEGF) gene polymorphisms on VEGF levels and the risk of vasoocclusive crisis in sickle cell disease. Eur J Haematol. 2012;89(5):403–9.
Zhai R, Gong MN, Zhou W, Thompson TB, Kraft P, Su L, et al. Genotypes and haplotypes of the VEGF gene are associated with higher mortality and lower VEGF plasma levels in patients with ARDS. Thorax. 2007;62:718–22.
Ferrante M, Pierik M, Henckaerts L, Joossens M, Claes K, Van Schuerbeek N, et al. The role of vascular endothelial growth factor (VEGF) in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:870–8.
Krippl P, Langsenlehner U, Renner W, Yazdani-Biuki B, Wolf G, Wascher TC, et al. A common 936 C/T gene polymorphism of vascular endothelial growth factor is associated with decreased breast cancer risk. Int J Cancer. 2003;106:468–71.
Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51:1635–9.
Petrovic MG, Korosec P, Kosnik M, Osredkar J, Hawlina M, Peterlin B, et al. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol Vis. 2008;14:1382–7.
Gardini AC, Faloppi L, Aprile G, Brunetti O, Caparello C, Corbelli J, et al. Multicenter prospective study of angiogenesis polymorphism validation in HCC patients treated with sorafenib. An INNOVATE study protocol. Tumori. 2018;104(6):476–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was used in the preparation of this article.
Conflict of interest
Luca Faloppi, Marco Puzzoni, Andrea Casadei Gardini, Nicola Silvestris, Gianluca Masi, Giorgia Marisi, Caterina Vivaldi, Cosmo Damiano Gadaleta, Pina Ziranu, Maristella Bianconi, Cristian Loretelli, Laura Demurtas, Eleonora Lai, Riccardo Giampieri, Eva Galizia, Paola Ulivi, Nicola Battelli, Alfredo Falcone, Stefano Cascinu and Mario Scartozzi declare that they have no conflicts of interest that might be relevant to the contents of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faloppi, L., Puzzoni, M., Casadei Gardini, A. et al. Angiogenesis Genotyping and Clinical Outcomes in Patients with Advanced Hepatocellular Carcinoma Receiving Sorafenib: The ALICE-2 Study. Targ Oncol 15, 115–126 (2020). https://doi.org/10.1007/s11523-020-00698-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11523-020-00698-x