Abstract
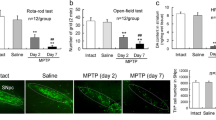
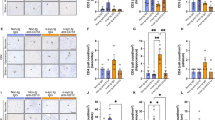
Regulatory T cells (Tregs), which secrete transforming growth factor (TGF)-β and interleukin (IL)-10, have essential role in anti-inflammatory and neurotrophic functions. Herein, we explore the neuroprotection of Tregs in Parkinson’s disease (PD) by adoptive transfer of Tregs. Tregs, isolated by magnetic sorting, were activated in vitro and then were adoptively transferred to 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP)-treated mice. Neuroinflammation, dopaminergic neuronal loss and behavioral changes of PD mice were evaluated. Live cell imaging system detected a dynamic contact of Tregs with MN9D cells that were stained with CD45 and galectin-1, respectively. Tregs prevented MPTP-induced dopaminergic neuronal loss, behavioral changes, and attenuated the inflammatory reaction in the brain. When blockade the LFA-1 activity in Tregs or the ICAM-1 activity in endothelial cells, the percentage of Tregs in substantia nigra (SN) decreased. CD45 and galectin-1 were expressed by Tregs and MN9D cells, respectively. CD45-labeled Tregs dynamically contacted with galectin-1-labeled MN9D cells. Inhibiting CD45 in Tregs impaired the ability of Tregs to protect dopaminergic neurons against MPP+ toxicity. Similarly, galectin-1 knockdown in MN9D cells reduced the ability of Tregs neuroprotection. Adoptive transfer of Tregs protects dopaminergic neurons in PD mice by a cell-to-cell contact mechanism underlying CD45-galectin-1 interaction.

Graphical Abstract








Similar content being viewed by others
References
Banerjee R, Mosley RL, Reynolds AD, Dhar A, Jackson-Lewis V, Gordon PH, Przedborski S, Gendelman HE (2008) Adaptive immune neuroprotection in G93A-SOD1 amyotrophic lateral sclerosis mice. PLoS One 3:e2740
Banks WA, Niehoff ML, Ponzio NM, Erickson MA, Zalcman SS (2012) Pharmacokinetics and modeling of immune cell trafficking: quantifying differential influences of target tissues versus lymphocytes in SJL and lipopolysaccharide-treated mice. J Neuroinflammation 9:231
Barcia C, Ros CM, Annese V, Gomez A, Ros-Bernal F, Aguado-Yera D, Martinez-Pagan ME, de Pablos V, Fernandez-Villalba E, Herrero MT (2011) IFNgamma signaling, with the synergistic contribution of TNF-alpha, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis 2:e142
Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, Buendia E (2001) Lymphocyte populations in Parkinson's disease and in rat models of parkinsonism. J Neuroimmunol 113:146–152
Bechmann I, Galea I, Perry VH (2007) What is the blood–brain barrier (not)? Trends Immunol 28:5–11
Benner EJ, Banerjee R, Reynolds AD, Sherman S, Pisarev VM, Tsiperson V, Nemachek C, Ciborowski P, Przedborski S, Mosley RL, Gendelman HE (2008) Nitrated alpha-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS One 3:e1376
Blom C, Deller BL, Fraser DD, Patterson EK, Martin CM, Young BM, Liaw PC, Yazdan-Ashoori P, Ortiz A, Webb B, Kilmer G, Carter DE, Cepinskas G (2015) Human severe sepsis cytokine mixture increases β2-integrin-dependent polymorphonuclear leukocyte adhesion to cerebral microvascular endothelial cells in vitro. Crit Care 19:149
Bohatschek M, Werner A, Raivich G (2001) Systemic LPS injection leads to granulocyte influx into normal and injured brain: effects of ICAM-1 deficiency. Exp Neurol 172:137–152
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119:182–192
Cao JJ, Li KS, Shen YQ (2011) Activated immune cells in Parkinson’s disease. J NeuroImmune Pharmacol 6:323–329
Cederbom L, Hall H, Ivars F (2000) CD4+CD25+ regulatory T cells downregulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol 30:1538–1543
Chung ES, Kim H, Lee G, Park S, Kim H, Bae H (2012) Neuro-protective effects of bee venom by suppression of neuroinflammatory responses in a mouse model of Parkinson’s disease: role of regulatory T cells. Brain Behav Immun 26:1322–1330
Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A (1996) Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration 5:137–143
Danikowski KM, Jayaraman S, Prabhakar BS (2017 Jun 9) Regulatory T cells in multiple sclerosis and myasthenia gravis. J Neuroinflammation 14(1):117. https://doi.org/10.1186/s12974-017-0892-8
Faucheux BA, Bonnet AM, Agid Y, Hirsch EC (1999) Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 353:981–982
Gee JM, Kalil A, Thullbery M, Becker KJ (2008) Induction of immunologic tolerance to myelin basic protein prevents central nervous system autoimmunity and improves outcome after stroke. Stroke 39:1575–1582
He J, Baum LG (2006) Endothelial cell expression of galectin-1 induced by prostate cancer cells inhibits T-cell transendothelial migration. Lab Investig 86:578–590
Huang Y, Liu Z, Cao BB, Qiu YH, Peng YP (2017) Treg cells protect dopaminergic neurons against MPP+ neurotoxicity via CD47-SIRPA interaction. Cell Physiol Biochem 41:1240–1254
Ishibashi S, Kuroiwa T, Sakaguchi M, Sun L, Kadoya T, Okano H, Mizusawa H (2007) Galectin-1 regulates neurogenesis in the subventricular zone and promotes functional recovery after stroke. Exp Neurol 207:302–313
Kosloski LM, Kosmacek EA, Olson KE, Mosley RL, Gendelman HE (2013) GM-CSF induces neuroprotective and anti-inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxicated mice. J Neuroimmunol 265:1–10
Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A (1999) MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson’s disease. Acta Neurobiol Exp 59:1–8
Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R (2009) Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 15:192–199
Lipowsky H (2012) The endothelial glycocalyx as a barrier to leukocyte adhesion and its mediation by extracellular proteases. Ann Biomed Eng 40:840–848
Liu Z, Chen HQ, Huang Y, Qiu YH, Peng YP (2016) Transforming growth factor-β1 acts via TβR-I on microglia to protect against MPP(+)-induced dopaminergic neuronal loss. Brain Behav Immun 51:131–143
Liu Z, Huang Y, Cao BB, Qiu YH, Peng YP (2017) Th17 cells induce dopaminergic neuronal death via LFA-1/ICAM-1 interaction in a mouse model of Parkinson’s disease. Mol Neurobiol 54:7762–7776
Long-Smith CM, Collins L, Toulouse A, Sullivan AM, Nolan YM (2010) Interleukin-1beta contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J Neuroimmunol 226:20–26
Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, and Gendelman HE (2009) Neuromodulatory Activities of CD4+CD25+ Regulatory T Cells in a Murine Model of HIV-1-Associated Neurodegeneration. The Journal of Immunology 182:3855-3865
Miklossy J, Doudet DD, Schwab C, Yu S, Mcgeer EG, McGeer PL (2006) Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp Neurol 197:275–283
Noack M, Miossec P (2014) Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev 13:668–677
Perillo NL, Pace KE, Seilhamer JJ, Baum LG (1995) Apoptosis of T cells mediated by galectin-1. Nature 378:736–739
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55:453–462
Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL (2007) Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J Leukoc Biol 82:1083–1094
Reynolds AD, Stone DK, Mosley RL, Gendelman HE (2009a) Proteomic studies of nitrated alpha-synuclein microglia regulation by CD4+CD25+ T cells. J Proteome Res 8:3497–3511
Reynolds AD, Stone DK, Mosley RL, Gendelman HE (2009b) Nitrated α-synucleininduced alterations in microglial immunity are regulated by CD4+ T cell subsets. J Immunol 182:4137–4149
Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE (2010) Regulatory T cells attenuate Th17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson's disease. J Immunol 184:2261–2271
Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L (2007) Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol 188:117–127
Sakaguchi S (2004) Naturally arising CD4+ regulatory t cells for immunologic selftolerance and negative control of immune responses. Annu Rev Immunol 22:531–562
Sakaguchi S, Yamaguchi T, Nomura T, Ono M (2008) Regulatory T cells and immune tolerance. Cell 133:775–787
Sasaguri K, Yamada K, Narimatsu Y, Oonuki M, Oishi A, Koda K, Kubo K, Yamamoto T, Kadoya T (2016) Stress-induced galectin-1 influences immune tolerance in the spleen and thymus by modulating CD45 immunoreactive lymphocytes. J Physiol Sci 67:489–496. https://doi.org/10.1007/s12576-016-0478-8
Savitt JM, Dawson VL, Dawson TM (2006) Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest 116:1744–1754
Singer BD, King LS, D'Alessio FR (2014) Regulatory T cells as immunotherapy. Front Immunol 5:46
Thornton AM, Shevach EM (2000) Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol 164:183–190
Tiemessen MM, Jagger AL, Evans HG, van Herwijnen MJ, John S, Taams LS (2007) CD4+CD25+Foxp3+ regulatory T cells induce alternative activation of human monocytes/macrophages. Proc Natl Acad Sci U S A 104:19446–19451
Varatharaj A, Galea I (2016) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12
Wang H, Sun J, Goldstein H (2008) Human immunodeficiency virus type 1 infection increases the in vivo capacity of peripheral monocytes to cross the blood-brain barrier into the brain and the in vivo sensitivity of the blood-brain barrier to disruption by lipopolysaccharide. J Virol 82:7591–7600
Yeh H, Moore DJ, Markmann JF, Kim JI (2013) Mechanisms of regulatory T cell counter-regulation by innate immunity. Transplant Rev (Orlando) 27:61–64
Acknowledgements
This work was supported by grants 31771293 and 81701633 from the National Natural Science Foundation of China, grant 18B13 from Nantong University, grant BK20180948 from Natural Science Foundation of Jiangsu Province of China, and a project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
All of the experiments were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee of Nantong University.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(AVI 55098 kb)
Rights and permissions
About this article
Cite this article
Huang, Y., Liu, Z., Cao, BB. et al. Treg Cells Attenuate Neuroinflammation and Protect Neurons in a Mouse Model of Parkinson’s Disease. J Neuroimmune Pharmacol 15, 224–237 (2020). https://doi.org/10.1007/s11481-019-09888-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-019-09888-5