Abstract
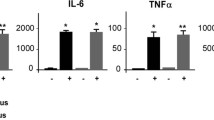
The purpose of the present study was to determine the specific role of the medial septal (MS) NMDA glutamate receptors on peripheral blood natural killer cell cytotoxicity (NKCC) and their (large granular lymphocyte, LGL) number, as well as the plasma concentration of tumor necrosis factor α (TNF-α) and corticosterone in male Wistar rats exposed to elevated plus maze (EPM) stress or non-stress conditions. The NMDA groups were injected with NMDA glutamate receptor agonist (N-methyl-D-aspartate; 0.25 μg/rat), the D-AP7 group was injected with DL-2-amino-7-phosphoheptanoate (0.1 μg/rat), an antagonist of NMDA glutamate receptors, and the control Sal group with saline (0.5 μl/rat) via previously implanted cannulae into the MS. There was an increase in the NKCC, NK/LGL number and plasma TNF-α concentration after the NMDA injections, being much stronger within the rats under non-stress conditions rather than the rats exposed to EPM stress. These parameters were decreased in the D-AP7 rats, suggesting receptor/ion channel specificity. Moreover, a lower plasma corticosterone concentration within the NMDA rather than the Sal and D-AP7 groups was found. The obtained results suggest that activation of the NMDA glutamate receptors in the MS, accompanied by changes in the corticosterone and cytokine responses, may be involved in modulation of the blood natural anti-tumor response, under EPM stress and non-stress conditions.





Similar content being viewed by others
References
Affaticati P, Mignen O, Jambou F, Potier MC, Klingel-Schmitt I, Degrouard J, Peineau S, Gouadon E, Collingridge GL, Liblau R, Capiod T, Cohen-Kaminsky S (2011) Sustained calcium signaling and caspase-3 activation involve NMDA receptors in thymocytes in contact with dendritic cells. Cell Death Differ 18:99–108
Becquet D, Hery M, Francois-Bellan AM, Giraud P, Deprez P, Faudon M, et al. (1993) Glutamate, GABA, glycine and taurine modulate serotonin synthesis and release in rostral and caudal rhombencephalic raphe cells in primary cultures. Neurochem Int 23:269–283
Boldyrev AA, Kazey VI, Leinsoo TA, Mashkina AP, Tyulina OV, Johnson P, Tuneva JO, Chittur S, Carpenter DO (2004) Rodent lymphocytes express functionally active glutamate receptors. Biochem Biophys Res Commun 324:133–139
Boldyrev AA, Bryushkova EA, Vladychenskaya EA (2012) NMDA receptors in immune competent cells. Biochem Mosc 77:128–134
Böyum A (1976) Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol 5:9–15
Cervera-Ferri A, Rahmani Y, Martinez-Bellver S, Teruel-Marti V, Martinez-Ricos (2012) Glutamatergic projection from the nucleus incertus to the septohippocampal system. Neurosci Lett 517:71–76.
Collard CD, Park KA, Montalto MC, Alapati S, Buras JA, Stahl GL, Colgan SP (2002) Neutrophil-derived glutamate regulates vascular endothelial barrier function. J Biol Chem 277:14801–14811
Cortese BM, Phan KL (2005) The role of glutamate in anxiety and related disorders. CNS Spectr 10:820–830
Cruz AP, Frei F, Graeff FG (1994) Ethopharmacological analysis of rat behavior on the elevated plus – maze. Pharmacol Biochem Behav 49:171–176
Dhabhar FS (2002) Stress-induced augmentation of immune function – the role of stress hormones, leukocyte trafficking and cytokines. Brain Behav Immun 16:785–798
Dingledine R, Borges K, Bowie D, Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51:7–61
Dorshkind K, Horseman ND (2000) The roles of prolactin, growth hormone, insulin-like growth factor-1, and thyroid hormones in lymphocyte development and function: insights from genetics models of hormone and hormone receptor deficiency. Endocr Rev 21:292–312
Dutta G, Goswami AR, Ghosh T (2013) Effects of stimulation of glutamate receptors in medial septum on some immune responses in rats. Brain Res 1538:116–125
Ganor Y, Besser M, Ben – Zakay N, Unger T, Levite M (2003) Human T cells express a functional ionotropic glutamate receptor GluR3, and glutamate by itself triggers integrin – mediated adhesion to laminin and fibronectin and chemotactic migration. J Immunol 170:4362–4372.
Ganor Y, Levite M (2014) The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J Neural Transm 121:983–1006
Gao M, Jin W, Qian Y, Ji L, Feng G, Sun J (2011) Effect of N – methyl – D – aspartate receptor antagonist on T helper cell differentiation induced by phorbol – myristate – acetate and ionomycin. Cytokine 56:458–465
Garg SK, Benerjee R, Kipnis J (2008) Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J Immunol 180:3866–3873
Hellstrand K, Hermodsson S, Strannegard O (1985) Evidence for a beta-adrenoceptor-mediated regulation of human natural killer cells. J Immunol 134:4095–4099
Herberman RB, Ortaldo JR (1981) Natural killer cells: their role in defences against disease. Science 214:24–30
Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y (2004) Glutamate signaling in peripheral tissues. Eur J Biochem 271:1–13
Hogg S (1996) A review of the validity and variability of the elevated plus – maze as an animal model of anxiety. Pharmacol Biochem Behav 54:21–30
Hosseini N, Nasehi M, Radahmadi M, Zarrindast MR (2013) Effects of CA1 glutamatergic systems upon memory impairments in cholestatic rats. Behav Brain Res 256:636–645
Itoh J, Nabeshima T, Kameyama T (1990) Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology 101:27–33
Jedema HP, Moghddam B (1996) Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem 66:1448–1453
Kaname H, Mori Y, Sumida Y, Kojima K, Kubo C, Tashiro N (2002) Changes in the leukocyte distribution and surface expression of adhesion molecules induced by hypothalamic stimulation in the cat. Brain Behav Immun 16:351–367
Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA (2005) HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12:878–892
Khakpai F, Nasehi M, Haeri – Rahani A, Eidi A, Zarrindast MR (2012) Scopolamine induced memory impairment, possible involvement of NMDA receptor mechanisms of dorsal hippocampus and/or septum. Behav Brain Res 231:1–10.
Khakpai F, Zarrindast MR, Nasehi M, Rohani AH, Eidi A (2013) The role of glutamatergic pathway between septum and hippocampus in the memory formation. EXCLI J 12:41–51
Kostanyan IA, Merkulova MI, Navolotskaya EV, Nurieva RI (1997) Study of interaction between L – glutamate and human blood lymphocytes. Immunol Lett 58:177–180
Kuo JS, Chen SF, Huang HJ, Yang CS, Tsai PJ, Hsueh CM (2001) The involvement of glutamate in recall of the conditioned NK cell response. J Neuroimmunol 118:245–255
Lamprea MR, Garcia AM, Morato S (2010) Effects of reversible inactivation of the medial septum on rat exploratory behavior in the elevated plus – maze using a test – retest paradigm. Behav Brain Res 210:67–73
Listowska M, Glac W, Grembecka B, Grzybowska M, Wrona D (2015) Changes in blood CD4+T and CD8+T lymphocytes in stressed rats pretreated chronically with desipramine are more pronounced after chronic open field stress challenge. J Neuroimmunol 282:54–62
Lombardi G, Dianzani C, Miglio C, Canonico PL, Fantozzi R (2001) Characterization of ionotropic glutamate receptors in human lymphocytes. Br J Pharmacol 133:936–944
Manseau F, Danik M, Williams S (2005) A functional glutamatergic neurone network in the medial septum and diagonal band area. J Physiol 566:865–884
Mashkina AP, Tyulina OV, Solovyova TI, Kovalenko EI, Kanevsky LM, Johnson P, Boldyrev AA (2007) The excitotoxic effect of NMDA on human lymphocyte immune function. Neurochem Int 51:356–360
Mashkina AP, Cizkova D, Vanicky I, Boldyrev AA (2010) NMDA receptors are expressed in lymphocytes activated both in vitro and in vivo. Cell Mol Neurobiol 30:901–907
Miglio G, Varsaldi F, Lombardi G (2005) Human T lymphocytes express N – methyl – D – aspartate receptors functionally active in controlling T cell activation. Biochem Res Commun 338:1875–1883
Nair A, Bonneau RH (2006) Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol 171:72–85
Pacheco R, Ciruela F, Casado V, Mallol J, Gallart T, Lluis C, Franco R (2004) Group I metabotropic glutamate receptors mediate a dual role of glutamate in T cell activation. J Biol Chem 279:33352–33358
Paxinos W, Watson C (2007) The rat brain in stereotaxic coordinates. Academic Press, New York
Pellow S, Chopin P, File S, Briley M (1985) Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14:149–167
Podlacha M, Myślińska D, Wrona D (2013) Immune response correlates with behavioral activity associated with memory processes following chronic stimulation of the medial septum. Eur Neuropsychopharmacol 23(Suppl. 2):S187–S188
Podlacha M, Grembecka B, Listowska M, Glac W, Majkutewicz I, Plucińska K, Wrona D (2014) Medial septal glutamatergic receptor antagonist decreases plasma tumor necrosis factor – alpha concentration in rats. Eur Neuropsychopharmacol 24(Suppl. 2):S196
Reijerkerk A, Kooij G, van der Pol S, Leyen T, Lakeman K, van het Hof B, Vivien D, de Vries H (2010) The NR1 subunit of NMDA receptor regulates monocyte transmigration through the brain endothelial cell barrier. J Neurochem 113:447–453
Rodgers RJ, Cao BJ, Dalvi A, Holmes A (1997) Animal model of anxiety: an ethological perspective. Braz J Med Biol Res 30:289–304
Skerry TM, Genever PG (2001) Glutamate signaling in non – neuronal tissues. Trends Pharmacol Sci 22:174–181
Storto M, De Grazia U, Battaglia G, Felli MP, Maroder M, Gullino A, Ragona G, Nicoletti F, Screpanti I, Frati L, Calogero A (2000) Expression of metabotropic glutamate receptors in murine thymocytes and thymic stromal cells. J Neuroimmunol 109:112–120
Timonen T, Reynolds C, Ortaldo JR, Herberman RB (1982) Isolation of human and rat natural killer cells. J Immunol Methods 51:269–277
Ulrich-LAI YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress response. Nat Rev Neurosci 10:397–409
Vizi ES, Mike A (2006) Nonsynaptic receptors for GABA and glutamate. Curr Top Med Chem 6:941–948
Vladychenskaya E, Tyulina O, Urano S, Boldyrev A (2011) Rat lymphocytes express NMDA receptors that take part in regulation of cytokine production. Cell Biochem Funct 7:527–533
Walf AA, Frye CA (2007) The use of the elevated plus maze as an assay of anxiety-elevated behavior in rodents. Nat Protoc 2:322–328
Wrona D, Trojniar W (2003) Chronic electrical stimulation of the lateral hypothalamus increases natural killer cell cytotoxicity in rats. J Neuroimmunol 141:20–29
Acknowledgments
This study was supported by Gdańsk University grants for young scientists from Poland: 538-L124-B074-13 and 538-L124-B597-14. The authors are grateful to Dr. Paweł Matulewicz for generously providing the isoflurane used in this study.
Conflict of Interest
Authors do not declare any conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podlacha, M., Glac, W., Listowska, M. et al. Medial Septal NMDA Glutamate Receptors are Involved in Modulation of Blood Natural Killer Cell Activity in Rats. J Neuroimmune Pharmacol 11, 121–132 (2016). https://doi.org/10.1007/s11481-015-9632-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11481-015-9632-y