Abstract
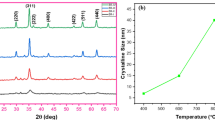
Fe3O4 and Fe3O4/Ag core–shell nanocomposite powders were synthesized via the co-precipitation method. The structure, microstructure, magnetic, and optical properties of Fe3O4/Ag and Fe3O4 were studied. XRD patterns and UV–Vis spectra showed that nanostructure Fe3O4 and Fe3O4/Ag particles were successfully synthesized. AFM and MFM mode micrographs of Fe3O4 and Fe3O4/Ag powders confirm the formation of Fe3O4 particles in the nano-scale range. Both Fe3O4 and Fe3O4/Ag composite powders represented the superparamagnetic behavior due to the formation of nanosized Fe3O4 particles. Furthermore, in-situ synthesis of Ag on the surface of magnetite nanoparticles increased the particle size, resulting in a decrease in saturation magnetization. Moreover, based on the Maxwell-Garnet effective medium theory, a theoretical model was developed to determine the optical properties of suspended core–shell nanoparticles. A very good agreement was found between the theoretical and experimental results. In addition, the local electric field in the particles, evaluated using the numerical finite element method, showed that the electric field in the magnetite core might be amplified up to 20 times at the symmetrical SPR wavelength mode, depending on the silver shell thickness.







Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Palpant B (2012) in Gold nanoparticles for physics, biology and chemistry, edited by Louis C and Pluchery O. (Imperial College Press, London, 2012), pp. 75–102. https://doi.org/10.1002/anie.201309807
Parisa K, Huyeh MR (2021). IJOP. https://doi.org/10.52547/ijop.15.1.41
Lesniak W, Bielinska AU, Sun K, Janczak KW, Shi X, Baker JR, Balogh LP (2005). Nano Lett. https://doi.org/10.1021/nl051077u
Liu CH, Zhou ZD, Yu X, Lv BQ, Mao JF, Xiao D (2005). AnalChem. https://doi.org/10.1134/S002016850803014X
Li K, Zhengtao Xu, Hanhui Xu, Carroll PJ, Fettinger JC (2006). Inorg Chem. https://doi.org/10.1021/ic051135e
Endo T, Yomokazu T, Esumi K (2005). J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2005.01.057
Tan LL, Wei M, Shang L, Yang YW (2021). Adv Funct Mater. https://doi.org/10.1002/adfm.202007277
Yang X, Yang M, Pang Bo, Vara M, Xia Y (2015). Chem Rev. https://doi.org/10.1021/acs.chemrev.5b00193
Arturo M, Lopez Q, Jose R (1993) J Colloid Interface Sci. https://doi.org/10.1006/jcis.1993.1277
Chiang CL, Sung CS (2006). J Magn Magn Mater. https://doi.org/10.1016/j.jmmm.2005.08.022
Aoshima K, Wang SX (2003). J Appl Phys. https://doi.org/10.1063/1.1558633
Morton SA, Waddill GD, Kim SI, Schuller K, Chambers SA, Tobin JG (2002) Surf Sci. https://doi.org/10.1016/s0039-6028(02)01824-1
Shebanova ON, Lazor P (2003) J Chem Phys. https://doi.org/10.1063/1.1602072
Schrupp D, Sing M, Tsunekawa M, Fujiwara H, Kasai S, Sekiyama A, Suga S, Muro T, Brabers VAM, Claessen R (2005). Europhys Lett. https://doi.org/10.1209/epl/i2005-10045-y
Huang ZB, Tang FQ (2004) Preparation, structure, and magnetic properties of polystyrene coated by Fe3O4 nanoparticles. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2003.12.065
Cui YL, Hu DD, Fang Y, Ma JB (2001) Sci China Ser B. https://doi.org/10.1007/BF02879815
Shimizu T, Kitayama Y, Kodama T (2001). Energy fuels. https://doi.org/10.1021/ef000200w
León Félix L, Coaquira JA, Martínez MA, Goya GF, Mantilla J, Sousa MH, Valladares LL, Barnes CH, Morais PC (2017) Scientific reports. https://doi.org/10.1038/srep41732
Salehizadeh H, Hekmatian E, Sadeghi M, Kennedy K (2012). J Nanobiotechnol. https://doi.org/10.1186/1477-3155-10-3
Brullot W, Valev VK, Verbiest T (2012) Nanomedicine: NBM. https://doi.org/10.1016/j.nano.2011.09.004
Wang G, Li F, Li L, Zhao J, Ruan X, Ding W, Cai J, Ang Lu, Pe Y (2020). ACS Omega. https://doi.org/10.1021/acsomega.0c00437
Khedkar CV, Khupse ND, Thombare BR, Dusane PR, Lole G, Devan RS, Deshpande AS, Patil SI (2020). Chem Phys Lett. https://doi.org/10.1016/j.cplett.2020.137131
Jiang W, Zhou Y, Zhang Y, Xuan S, Gong X (2012). Dalton Trans. https://doi.org/10.1039/C2DT12307J
Chen Q, Wang H, Wang Q, Pan Y (2018) J Plasmonics. https://doi.org/10.1051/e3sconf/201911801002
Amarjargal A, Tijing LD, Ik-Tae I, Kim CS (2013) Chemical Engineering Journal. https://doi.org/10.1016/j.cej.2013.04.054
Benvidi A, Jahanbani S (2016) J Electroanalytic Chem. https://doi.org/10.1016/j.jelechem.2016.02.038
Liu CH, Zhou ZD, Xiao D (2008) Inorganic materials. https://doi.org/10.1134/S002016850803014X
Ghazanfari M, Johar F, Yazdani A (2014) Journal of ultrafine grained and nanostructured materials. https://doi.org/10.7508/jufgnsm.2014.02.006
Bankole OM, Nyokong T (2016). New J Chem. https://doi.org/10.1039/C6NJ01511E
Shan Y, Yang Y, Caoab Y, Huang Z (2015). RSC Adv. https://doi.org/10.1039/C5RA17606A
Gai K, Qi H, Zhu X, Wan M (2019) E3S Web Conf. https://doi.org/10.1051/e3sconf/201911801002
Pan L, Tang J, Chen Y (2013). Sci China Chem. https://doi.org/10.1007/s11426-012-4763-y
Amarjargal A, Tijing LD, Cheol I-T, Kim S (2013). Chem Eng J. https://doi.org/10.1016/j.cej.2013.04.054
Khalil MI (2015). Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.02.008
Wang G, Li F, Li L, Zhao J, Ruan X, Ding W, Cai J, Ang Lu, Pei Y (2020). ACS Omega. https://doi.org/10.1021/acsomega.0c00437
Fajaroh F (2017) IOP Conf. Ser.: Mater Sci Eng. https://doi.org/10.1088/1757-899X/202/1/012064
Ianoș R, Tăculescu A, Păcurariu C, Lazău I (2012) J Am Ceram Soc. https://doi.org/10.1111/j.1551-2916.2012.05159.x
Tahmasebi E, Yamini Y (2012) Anal Chim Acta. https://doi.org/10.1016/j.aca.2012.10.040
Patsula V, Kosinová L, Lovrić M, Ferhatovic Hamzić L, Rabyk M, Konefal R, Paruzel A, Šlouf M, Herynek V, Gajović S, Horák D (2016) ACS Appl Mater & Interf. https://doi.org/10.1021/acsami.5b12720
Villegas VRA, Isaías De León Ramírez J, Hernandez Guevara E, Sicairos SP, Hurtado Ayala LA, Sanchez BL (2020) J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2019.12.004
Hashemi Zadeh S, Rashidi-Huyeh M, Palpant B (2017) J Appl Phys. https://doi.org/10.1063/1.4997276
Prodan E, Radloff C, Halas NJ, Nordlander P (2003) Science. https://doi.org/10.1126/science.1089171
Prodan E, Nordlande P (2004). J Chem Phys. https://doi.org/10.1063/1.1647518
Voshchinnikov NV, Mathis JS (1999) Astrophys J 526:257–264
Levin CS, Hofmann C, Ali TA, Kelly AT, Morosan E, Nordlander P, Whitmire KH, Halas NJ (2009) ACS Nano. https://doi.org/10.1021/nn900118a
Acknowledgements
The authors would like to thank Mohammad Azari Nadjaf Abad from the Karlsruhe Institute of Technology and Anh Van Le from the University of Tuebingen for their scientific comments.
Funding
This work was supported by the University of Sistan and Baluchestan, Iran. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by Majid Rashidi Huyeh, Saeideh Balouchzahi, Mahdi Shafiee Afarani, and Parisa Khajegi. The first draft of the manuscript was written by Majid Rashidi Huyeh and Mahdi Shafiee Afarani, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rashidi Huyeh, M., Balouchzehi, S., Shafiee Afarani, M. et al. Magnetite-Silver Core–Shell Nanoparticles: Synthesis, Characterizes, and Optical Properties. Plasmonics 17, 2385–2390 (2022). https://doi.org/10.1007/s11468-022-01725-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11468-022-01725-5