Abstract
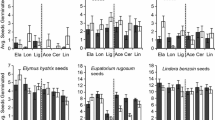
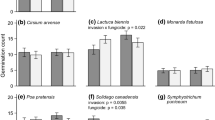
Soil microbes are one of the most important determinants of allelopathic effects in the field. However, most studies testing the role of allelopathy in biological invasions did not consider the roles of soil microbes. Here we tested the hypothesis that soil microbes which can degrade allelochemicals may accumulate in soils over time by adaptation and therefore increase the degradation of allelochemicals and alleviate the allelopathic effects in biological invasions. As expected, soil microbes significantly decreased the allelopathic effects of leaf leachates of eight in the nine invasive plant species studied. In addition, Ageratina adenophora showed lower allelopathic effects in soil with long or intermediately invasion history than those in soil with short invasion history. The two main allelochemicals of the invader were degraded more rapidly with increasing invasion history in the soil. Correspondingly, biomass and activity of the soil microbes were higher in the soils with long invasion history than in that with short invasion history. Our results indicate that soil microbes may gradually adapt to the allelochemicals of Ageratina and alleviate its allelopathic effects and thus support the above hypothesis. It is necessary to consider the effects of soil microbes when testing the roles of allelopathy or the novel weapons hypothesis in biological invasions.



Similar content being viewed by others
References
Callaway RM, Ridenour W (2004) Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ 2:436–443
Murrell C, Gerber E, Krebs C et al (2011) Invasive knotweed affects native plants through allelopathy. Am J Bot 98:38–43
Smith LM, Reynolds HL (2014) Light, allelopathy, and post-mortem invasive impact on native forest understory species. Biol Invasions 16:1131–1144
Zheng L, Feng YL (2005) Allelopathic effects of Eupatorium adenophorum Spreng. on seed germination and seedling growth in ten herbaceous species. Acta Ecol Sin 25:2782–2787
Zheng YL, Feng YL, Zhang LK et al (2015) Integrating novel chemical weapons and evolutionarily increased competitive ability in success of a tropical invader. New Phytol 205:1350–1359
Callaway RM, Aschehoug ET (2000) Invasive plants versus their new and old neighbors: a mechanism for exotic invasion. Science 290:521–523
Inderjit, Evans H, Crocoll C (2011) Volatile chemicals from leaf litter are associated with invasiveness of a neotropical weed in Asia. Ecology 92:316–324
Qin RM, Zheng YL, Valiente-Banuet A (2013) The evolution of increased competitive ability, innate competitive advantages, and novel biochemical weapons act in concert for a tropical invader. New Phytol 197:979–988
Inderjit, Nilsen ET (2003) Bioassays and field studies for allelopathy in terrestrial plants: progress and problems. Crit Rev Plant Sci 22:231–238
Tharayil N, Bhowmik P, Alpert P et al (2009) Dual purpose secondary compounds: phytotoxin of Centaurea diffusa also facilitates nutrient uptake. New Phytol 181:424–434
Metlen KL, Aschehoug ET, Callaway RM (2013) Competitive outcomes between two exotic invaders are modified by direct and indirect effects of a native conifer. Oikos 122:632–640
Asaduzzaman M, An M, Pratley JE et al (2014) Canola (Brassica napus) germplasm shows variable allelopathic effects against annual ryegrass (Lolium rigidum). Plant Soil 380:47–56
Blair A, Hanson B, Brunk G et al (2005) New techniques and findings in the study of a candidate allelochemical implicated in invasion success. Ecol Lett 8:1039–1047
Callaway RM, Cipollini D, Barto K et al (2008) Novel weapons: invasive plant suppresses fungal mutualists in America but not in its native Europe. Ecology 89:1043–1055
Weidenhamer J, Romeo J (2004) Allelochemicals of Polygonella myriophylla: chemistry and soil degradation. J Chem Ecol 30:1067–1082
Inderjit (2005) Soil microorganisms: an important determinant of allelopathic activity. Plant Soil 274:227–236
Cipollini D, Rigsby CM, Barto EK (2012) Microbes as targets and mediators of allelopathy in plants. J Chem Ecol 38:714–727
Ehlers BK (2011) Soil microorganisms alleviate the allelochemical effects of a Thyme Monoterpene on the performance of an associated grass species. PLoS One 6:e26321
Blum U (1998) Effects of microbial utilization of phenolic acids and their phenolic acid breakdown products on allelopathic interactions. J Chem Ecol 24:685–708
Chen L, Yang X, Raza W et al (2011) Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl Microbiol Biotechnol 89:1653–1663
Arbeli Z, Fuentes CL (2007) Accelerated biodegradation of pesticides: an overview of the phenomenon, its basis and possible solutions, and a discussion on the tropical dimension. Crop Prot 26:1733–1746
Lawrence JG, Ochman H (2002) Reconciling the many faces of lateral gene transfer. Trends Microbiol 10:1–4
Top EM, Springael D, Boon N (2002) Catabolic mobile genetic elements and their potential use in bioaugmentation of polluted soils and waters. FEMS Microbiol Ecol 42:199–208
Aelion CM, Swindoll CM, Pfaender FK (1987) Adaptation to and biodegradation of xenobiotic compounds by microbial communities from a pristine aquifer. Appl Environ Microbiol 53:2212–2217
Gray RA, Joo GK (1985) Reduction in weed control after repeated application of thiocarbamate and other herbicides. Weed Sci 33:698–702
Karpouzas DG, Hatziapostolou P, Papadopoulou-Mourkidou E et al (2004) The enhanced biodegradation of fenamiphos in soils from previously treated sites and the effect of soil fumigants. Environ Toxicol Chem 23:2099–2107
Perry LG, Thelen GC, Ridenour WM et al (2005) Dual role for an allelochemical: (±)-catechin from Centaurea maculosa root exudates regulates conspecific seedling establishment. J Ecol 93:1126–1135
Perry LG, Thelen GC, Ridenour WM et al (2007) Concentrations of the allelochemical (±)-catechin in Centaurea maculosa soils. J Chem Ecol 33:2337–2344
Inderjit, Pollock JL, Callaway RM et al (2008) Phytotoxic effects of (±)-catechin in vitro, in soil, and in the field. PLoS One 3:e2536
Kaur H, Kaur R, Kaur S et al (2009) Taking ecological function seriously: soil microbial communities can obviate allelopathic effects of released metabolites. PLoS One 4:e4700
Vance ED, Brookes PC, Jenkinson DS (1987) Microbial biomass measurements in forest soils: determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol Biochem 19:689–696
Yang G, Wan F, Liu W et al (2008) Influence of two allelochemicals from Ageratina adenophora Sprengel on ABA, IAA and ZR contents in roots of upland rice seedlings. Allelopathy J 21:253
Williamson B, Richardson D (1988) Bioassays for allelopathy: measuring treatment responses with independent controls. J Chem Ecol 14:181–187
Li ZG, Luo YM, Teng Y (2008) Methods of analysis soil and environment microbes. Science Press, Beijing
Lankau RA (2010) Soil microbial communities alter allelopathic competition between Alliaria petiolata and a native species. Biol Invasions 12:2059–2068
Zhu X, Zhang J, Ma KP (2011) Soil biota reduce allelopathic effects of the invasive Eupatorium adenophorum. PLoS One 6:e25393
Meiners SJ, Kong CH, Ladwig LM et al (2012) Developing an ecological context for allelopathy. Plant Ecol 213:1861–1867
Hole SJ, McClure NC, Powles SB (2001) Rapid degradation of carbetamide upon repeated application to Australian soils. Soil Biol Bioch 33:739–745
Lau JA (2006) Evolutionary responses of native plants to novel community members. Evolution 60:56–63
Kong CH, Xu XH, Chen JJ et al (2002) Allelopathy of Ageratum conyzoides IX. Transformation of main allelochemical in the soil. Acta Ecol Sin 8:1189–1195
Lalljee B, Facknath S (2000) Allelopathic interactions in soil. In: Narwal SS et al (eds) Allelopathy in ecological agriculture and forestry. Kluwer Academic Publishers, Dordrecht, pp 47–58
Karban R (2007) Experimental clipping of sagebrush inhibits seed germination of neighbours. Ecol Lett 10:791–797
Rasher DB, Hay ME (2014) Competition induces allelopathy but suppresses growth and anti-herbivore defence in a chemically rich seaweed. Proc R Soc B 281:20132615
Acknowledgments
We are grateful to Da-Wen Li and Ailaoshan Station for Subtropical Forest Ecosystem Studies, Chinese Academy of Sciences for field assistance. This work was supported by the National Natural Science Foundation of China (31100410, 31470575 and 30830027), the National Key Technology R&D Program of China (2011BAD30B00), and Chinese Academy Science 135 Program (XTBG-T01, F01).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, YP., Feng, YL., Chen, YJ. et al. Soil microbes alleviate allelopathy of invasive plants. Sci. Bull. 60, 1083–1091 (2015). https://doi.org/10.1007/s11434-015-0819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-015-0819-7