Abstract
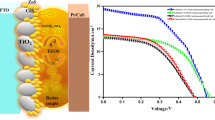
Two organic sulfide redox couples derived from 2-mercapto-5-methyl-1,3,4-thiadiazole (McMT): tetrabutylammonium thiolate (McMT−TBA+)/disulfide dimer (BMT) and tetramethylammonium thiolate (McMT−TMA+)/BMT were incorporated into quantum dots sensitized solar cells (QDSCs) as alternatives to the inorganic polysulfide electrolyte (Na2S/S). It was found in symmetrical cells test that the interfaces of the organic sulfide electrolytes/platinum counter-electrode have much lower charge transfer resistances as well as higher interface reaction rates compared with that for the inorganic one. Besides, QDSCs based on organic sulfide electrolytes exhibited obviously higher fill factors, open circuit photovoltages, and therefore higher conversion efficiency, thanks to the prohibited recombination and lower redox potential. In addition, by comparing and analyzing the performance of devices based on organic sulfide electrolytes with different cationic groups, it is found that cationic group TMA+ with smaller size was favorable for the mass transport in the electrolyte, which explains the better photovoltaic performance of McMT−TMA+/BMT based solar cell than that of McMT−TBA+/BMT based one. Eventually, a power conversion efficiency (PCE) of 0.63 % was achieved for QDSCs using McMT−(TMA+)/BMT redox couple as electrolyte, which was till now the highest for CdS QDSC based on organic sulfide electrolyte.







Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Hagfeldt A, Boschloo G, Sun L et al (2010) Dye-sensitized solar cells. Chem Rev 110:6595–6663
Wei D (2010) Dye sensitized solar cells. Int J Mol Sci 11:1103–1113
Grätzel M (2004) Conversion of sunlight to electric power by nanocrystalline dye-sensitized solar cells. J Photoch Photobio A 164:3–14
Jun HK, Careem MA, Arof AK (2013) A suitable polysulfide electrolyte for CdSe quantum dot-sensitized solar cells. Int J Photoenergy 2013: ID 942139
Grätzel M (2005) Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 44:6841–6851
Rühle S, Shalom M, Zaban A (2010) Quantum-dot-sensitized solar cells. ChemPhysChem 11:2290–2304
Shen Q, Kobayashi J, Diguna LJ et al (2008) Effect of ZnS coating on the photovoltaic properties of CdSe quantum dot-sensitized solar cells. J Appl Phys 103:084304
Baker DR, Kamat PV (2009) Photosensitization of TiO2 nanostructures with CdS quantum dots: particulate versus tubular support architectures. Adv Funct Mater 19:805–811
Shen H, Jiao X, Oron D et al (2013) Efficient electron injection in non-toxic silver sulfide (Ag2S) sensitized solar cells. J Power Sources 240:8–13
Yang ZS, Chen CY, Roy P et al (2011) Quantum dot-sensitized solar cells incorporating nanomaterials. Chem Commun 47:9561–9571
Im JH, Lee CR, Lee JW et al (2011) 6.5 % efficient perovskite quantum-dot-sensitized solar cell. Nanoscale 3:4088–4093
Yella A, Lee H, Tsao H et al (2011) Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 334:629
Li L, Yang X, Gao J et al (2011) Highly efficient CdS quantum dots sensitized solar cells based on modified polysulfide electrolyte. J Am Chem Soc 133:8458–8460
Ning Z, Tian H, Yuan C, Fu Y et al (2011) Pure organic redox couple for quantum-dot-sensitized solar cells. Chemistry 17:6330–6333
Lee YL, Chang CH (2008) Efficient polysulfide electrolyte for CdS quantum dot-sensitized solar cells. J Power Sources 185:584–588
Kim J, Choi H, Nahm C et al (2011) The effect of a blocking layer on the photovoltaic performance in CdS quantum-dot-sensitized solar cells. J Power Sources 196:10526–10531
Lee HJ, Chen P, Moon SJ et al (2009) Regenerative PbS and CdS quantum dot sensitized solar cells with a cobalt complex as hole mediator. Langmuir 25:7602–7608
Wang M, Chamberland N, Breau LE et al (2010) An organic redox electrolyte to rival triiodide/iodide in dye-sensitized solar cells. Nat Chem 2:385–389
Tian H, Jiang X, Yu Z et al (2010) Efficient organic-dye-sensitized solar cells based on an iodine-free electrolyte. Angew Chem Int Edit 49:7328–7331
Shen H, Lin H, Liu Y et al (2011) A novel diphenylphosphinic acid coadsorbent for dye-sensitized solar cell. Electrochim Acta 56:2092–2097
Nicolau YF, Dupuy M, Brunel M (1990) ZnS, CdS, and Zn1−x Cd x S thin films deposited by the successive ionic layer adsorption and reaction process. J Electrochem Soc 137:2915–2924
Zhang Q, Zhang Y, Huang S et al (2010) Application of carbon counterelectrode on CdS quantum dot-sensitized solar cells (QDSSCs). Electrochem Commun 12:327–330
Lin SC, Lee YL, Chang CH et al (2007) Quantum-dot-sensitized solar cells: assembly of CdS-quantum-dots coupling techniques of self-assembled monolayer and chemical bath deposition. Appl Phys Lett 90:143517
Lessner PM, McLarnon FR, Winnick J et al (1993) The dependence of aqueous sulfur-polysulfide redox potential on electrolyte composition and temperature. J Electrochem Soc 140:1847–1849
Liu M, Visco SJ, Jonghe LC (1989) Electrochemical properties of organic disulfide/thiolate redox couples. J Electrochem Soc 136:2570–2575
Itzhakov S, Shen H, Buhbut S et al (2012) Quantum dot-sensitized solar cell spanning the visible and near-infrared spectrum. J Phys Chem C 117:22203–22210
Tachan Z, Shalom M, Hod I et al (2011) PbS as a highly catalytic counter electrode for polysulfide-based quantum dot solar cells. J Phys Chem C 115:6162–6166
Hodes G, Manassen J, Cahen D (1980) Electrocatalytic electrodes for the polysulfide redox system. J Electrochem Soc 127:544–549
Hao F, Dong P, Zhang J et al (2012) High electrocatalytic activity of vertically aligned single-walled carbon nanotubes towards sulfide redox shuttles. Sci Rep 2:368
Acknowledgments
This work was supported by the MOST International S&T Cooperation Program of China (2010DFA64360), the Ministry of Science & Technology, Israel and the Ministry of Science & Technology, China, the China-Israel Scientific and Strategic Research Fund-No. 7 of the 5th Round and the 6th Round (2013DFG53010), and the National Natural Science Foundation of China (51272126).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xin Li and Heping Shen has contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, X., Shen, H., Wang, W. et al. Effect of cationic groups in organic sulfide electrolyte on the performance of CdS quantum dot sensitized solar cells. Chin. Sci. Bull. 59, 3209–3215 (2014). https://doi.org/10.1007/s11434-014-0416-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11434-014-0416-1