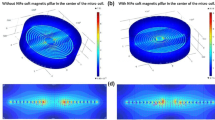
Abstract
Immunomagnetic bead (IMB)-based detection has great potential for biomedical applications. Passive and active strategies, including microfluidics and magnetic actuation methods, have been developed to mix IMBs and analytes efficiently. However, cost-effective on-site detection using a simple microfluidic chip is challenging, and miniaturization of the magnetic driving device is imperative for portability. In this study, we propose a novel mixing method for an on-chip IMB swarm via magnetic actuation and mechanical vibration. A microfluidic chip system coupled with double spiral magnetic coils and a vibration motor was fabricated. The aggregation behavior of IMBs under magnetic fields and the diffusion behavior of the IMB swarm under mechanical vibration were analyzed in detail. Based on the synergetic effects of magnetic actuation and mechanical vibration, we achieved the highly efficient capturing of Vibrio parahaemolyticus DNA and goat anti-human immunoglobulin G by mixing the IMB swarm with the microfluidic chip. In this case, the antigen detection rate could reach ∼94.4%. Given its fascinating features, such IMB-microfluidic detection demonstrates significant potential for biomedical applications.
Similar content being viewed by others
References
Wang L, Wang X, Cheng L, et al. SERS-based test strips: Principles, designs and applications. Biosens Bioelectron, 2021, 189: 113360
Cinti S, Moscone D, Arduini F. Preparation of paper-based devices for reagentless electrochemical (bio)sensor strips. Nat Protoc, 2019, 14: 2437–2451
Byram C, Soma V R. 2,4-dinitrotoluene detected using portable Raman spectrometer and femtosecond laser fabricated Au-Ag nanoparticles and nanostructures. Nano-Struct Nano-Objects, 2017, 12: 121–129
Westphall M S, Lee K W, Salome A Z, et al. Three-dimensional structure determination of protein complexes using matrix-landing mass spectrometry. Nat Commun, 2022, 13: 2276
Yeh E C, Fu C C, Hu L, et al. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci Adv, 2017, 3: e1501645
Shang Y, Xiang X, Ye Q, et al. Advances in nanomaterial-based microfluidic platforms for on-site detection of foodborne bacteria. TrAC Trends Anal Chem, 2022, 147: 116509
Xu D, Huang X, Guo J, et al. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens Bioelectron, 2018, 110: 78–88
Vollertsen A R, de Boer D, Dekker S, et al. Modular operation of microfluidic chips for highly parallelized cell culture and liquid dosing via a fluidic circuit board. Microsyst Nanoeng, 2020, 6: 107
Zheng H, Zhang W, Yang H, et al. An immunomagnetic bead enrichment technique to improve the detection efficiency for trace silk protein, its application. J Cultural Heritage, 2019, 38: 46–52
Wang X, He X, He Z, et al. Detection of prostate specific antigen in whole blood by microfluidic chip integrated with dielectrophoretic separation and electrochemical sensing. Biosens Bioelectron, 2022, 204: 114057
Ma Y D, Chen Y S, Lee G B. An integrated self-driven microfluidic device for rapid detection of the influenza A (H1N1) virus by reverse transcription loop-mediated isothermal amplification. Sens Actuat B-Chem, 2019, 296: 126647
Groisman A, Quake S R. A microfluidic rectifier: Anisotropic flow resistance at low Reynolds numbers. Phys Rev Lett, 2004, 92: 94501
Duong H L, Chen P C. Novel solvent bonding method for creation of a three-dimensional, non-planar, hybrid PLA/PMMA microfluidic chip. Sens Actuat A-Phys, 2018, 280: 350–358
Chen H. A triplet parallelizing spiral microfluidic chip for continuous separation of tumor cells. Sci Rep, 2018, 8: 4042
Xu B, Du W Q, Li J W, et al. High efficiency integration of three-dimensional functional microdevices inside a microfluidic chip by using femtosecond laser multifoci parallel microfabrication. Sci Rep, 2016, 6: 19989
Liu P, Li B, Fu L, et al. Hybrid three dimensionally printed paper-based microfluidic platform for investigating a cell’s apoptosis and intracellular cross-talk. ACS Sens, 2020, 5: 464–473
Du M, Ma Z S, Ye X Y, et al. On-chip fast mixing by a rotary peristaltic micropump with a single structural layer. Sci China Tech Sci, 2013, 56: 1047–1054
Yu J, Yang L, Zhang L. Pattern generation and motion control of a vortex-like paramagnetic nanoparticle swarm. Int J Robot Res, 2018, 37: 912–930
Magdanz V, Khalil I S M, Simmchen J, et al. IRONSperm: Spermtemplated soft magnetic microrobots. Sci Adv, 2020, 6: a5855
Yigit B, Alapan Y, Sitti M. Programmable collective behavior in dynamically self-assembled mobile microrobotic swarms. Adv Sci, 2019, 6: 1801837
Xin C, Yang L, Li J, et al. Conical hollow microhelices with superior swimming capabilities for targeted cargo delivery. Adv Mater, 2019, 31: 1808226
Feng Z, Zhi S, Guo L, et al. A novel integrated microfluidic platform based on micro-magnetic sensor for magnetic bead manipulation and detection. Microfluid Nanofluid, 2018, 22: 86
Gooneratne C P, Liang C, Kosel J. A planar conducting microstructure to guide and confine magnetic beads to a sensing zone. Microelectron Eng, 2011, 88: 1757–1760
Issadore D, Franke T, Brown K A, et al. A microfluidic microprocessor: Controlling biomimetic containers and cells using hybrid integrated circuit/microfluidic chips. Lab Chip, 2010, 10: 2937
Silverio V, Amaral M, Gaspar J, et al. Manipulation of magnetic beads with thin film microelectromagnet traps. Micromachines, 2019, 10: 607
Wu X Y, Wu H Y, Hu D H. High-efficiency magnetophoretic separation based on synergy of magnetic force field and flow field in microchannels. Sci China Tech Sci, 2011, 54: 3311–3319
Qi W, Zheng L, Wang S, et al. A microfluidic biosensor for rapid and automatic detection of Salmonella using metal-organic framework and Raspberry Pi. Biosens Bioelectron, 2021, 178: 113020
Ren Y, Ray S, Liu Y. Reconfigurable acrylic-tape hybrid microfluidics. Sci Rep, 2019, 9: 4824
Weng C H, Lien K Y, Yang S Y, et al. A suction-type, pneumatic microfluidic device for liquid transport and mixing. Microfluid Nanofluid, 2011, 10: 301–310
López R R, Sánchez L M, Alazzam A, et al. Numerical and experimental validation of mixing efficiency in periodic disturbance mixers. Micromachines, 2021, 12: 1102
Wang L, Niu J, Wei P, et al. Rapid determination of 2,4-diaminopyrimidine residues through sample pretreatment using immunomagnetic bead purification along with HPLC-UV. Food Chem, 2022, 376: 131835
Kuciński K, Jankowska-Wajda M, Ratajczak T, et al. Silica surface modification and its application in permanent link with nucleic acids. ACS Omega, 2018, 3: 5931–5937
de Lambert B, Chaix C, Charreyrex M T, et al. Polymer-oligonucleotide conjugate synthesis from an amphiphilic block copolymer. Applications to DNA detection on microarray. Bioconjugate Chem, 2005, 16: 265–274
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant No. 51975574) and the Fundamental Research Funds for the Central Universities (Grant No. 2020TC017).
Rights and permissions
About this article
Cite this article
Pan, J., Gong, D., Saeed, R. et al. On-chip immunomagnetic bead swarm based on magnetic actuation and mechanical vibration for biological detection. Sci. China Technol. Sci. 65, 2573–2581 (2022). https://doi.org/10.1007/s11431-022-2169-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-022-2169-6