Abstract
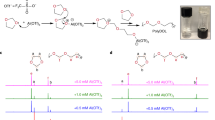
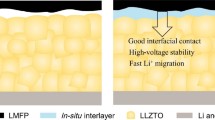
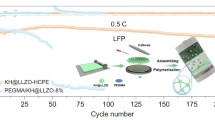
The practical applications of solid-state electrolytes in lithium-ion batteries (LIBs) are hindered by their low ionic conductivity and high interfacial resistance. Herein, an ethoxylated trimethylolpropane triacrylate based quasi-solid-state electrolyte (ETPTA-QSSE) with a three-dimensional (3D) network is prepared by a one-step in-situ photopolymerization method. The 3D network is designed to overcome the contradiction between the plasticizer-related ionic conductivity and the thickness-dependent mechanical property of quasi-solid-state electrolytes. The ETPTA-QSSE achieves superb room-temperature ionic conductivity up to 4.55×10−3 S cm−1, a high lithium ion transference number of 0.57, along with a wide electrochemical window of 5.3 V (vs. Li+/Li), which outperforms most ever of the reported solid-state electrolytes. Owing to the robust network structure and the cathode-electrolyte integrated electrode design, Li metal symmetrical cells show reduced interface resistance and reinforced electrode/ electrolyte interface stability. When applying the ETPTA-QSSE in LiFePO4∥Li cells, the quasi-solid-state cell demonstrates an enhanced initial discharge capacity (155.5 mAh g−1 at 0.2 C) accompanied by a high average Coulombic efficiency of greater than 99.3%, offering capacity retention of 92% after 200 cycles. Accordingly, this work sheds light on the strategy of enhancing ionic conductivity and reducing interfacial resistance of quasi-solid-state electrolytes, which is promising for high-voltage LIBs.
Similar content being viewed by others
References
Goodenough J B, Kim Y. Challenges for rechargeable Li batteries. Chem Mater, 2009, 22: 587–603
Goodenough J B, Park K S. The Li-ion rechargeable battery: A perspective. J Am Chem Soc, 2013, 135: 1167–1176
Zhou D, Shanmukaraj D, Tkacheva A, et al. Polymer electrolytes for lithium-based batteries: Advances and prospects. Chem, 2019, 5: 2326–2352
Wan J, Xie J, Kong X, et al. Ultrathin, flexible, solid polymer composite electrolyte enabled with aligned nanoporous host for lithium batteries. Nat Nanotechnol, 2019, 14: 705–711
Hatzell K B, Chen X C, Cobb C L, et al. Challenges in lithium metal anodes for solid-state batteries. ACS Energy Lett, 2020, 5: 922–934
Ge J, Fan L, Rao A M, et al. Surface-substituted prussian blue analogue cathode for sustainable potassium-ion batteries. Nat Sustain, 2022, 5: 225–234
Zhang Q, Liu K, Ding F, et al. Recent advances in solid polymer electrolytes for lithium batteries. Nano Res, 2017, 10: 4139–4174
Manthiram A, Yu X, Wang S. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater, 2017, 2: 16103
Zhang J, Zhao N, Zhang M, et al. Flexible and ion-conducting membrane electrolytes for solid-state lithium batteries: Dispersion of garnet nanoparticles in insulating polyethylene oxide. Nano Energy, 2016, 28: 447–454
Shi Y, Liu G X, Wan J, et al. In-situ nanoscale insights into the evolution of solid electrolyte interphase shells: Revealing interfacial degradation in lithium metal batteries. Sci China Chem, 2021, 64: 734–738
Jiang T, He P, Wang G, et al. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solidstate lithium batteries. Adv Energy Mater, 2020, 10: 1903376
Zhao Y, Wang L, Zhou Y, et al. Solid polymer electrolytes with high conductivity and transference number of Li ions for Li-based rechargeable batteries. Adv Sci, 2021, 8: 2003675
Oh D Y, Nam Y J, Park K H, et al. Excellent compatibility of solvate ionic liquids with sulfide solid electrolytes: Toward favorable ionic contacts in bulk-type all-solid-state lithium-ion batteries. Adv Energy Mater, 2015, 5: 1500865
Cheng E J, Kimura T, Shoji M, et al. Ceramic-based flexible sheet electrolyte for Li batteries. ACS Appl Mater Interfaces, 2020, 12: 10382–10388
Fan W, Li N W, Zhang X, et al. A dual-salt gel polymer electrolyte with 3D cross-linked polymer network for dendrite-free lithium metal batteries. Adv Sci, 2018, 5: 1800559
Ding C, Huang L, Guo Y, et al. An ultra-durable gel electrolyte stabilizing ion deposition and trapping polysulfides for lithium-sulfur batteries. Energy Storage Mater, 2020, 27: 25–34
Al-Masri D, Yunis R, Zhu H, et al. A new approach to very high lithium salt content quasi-solid state electrolytes for lithium metal batteries using plastic crystals. J Mater Chem A, 2019, 7: 25389–25398
Tan R, Gao R, Zhao Y, et al. Novel organic-inorganic hybrid electrolyte to enable LiFePO4 quasi-solid-state Li-ion batteries performed highly around room temperature. ACS Appl Mater Interfaces, 2016, 8: 31273–31280
Jiang G, Qu C, Xu F, et al. Glassy metal-organic-framework-based quasi-solid-state electrolyte for high-performance lithium-metal batteries. Adv Funct Mater, 2021, 31: 2104300
Zhou J, Ji H, Liu J, et al. A new high ionic conductive gel polymer electrolyte enables highly stable quasi-solid-state lithium sulfur battery. Energy Storage Mater, 2019, 22: 256–264
Zhou M, Liu R, Jia D, et al. Ultrathin yet robust single lithium-ion conducting quasi-solid-state polymer-brush electrolytes enable ultra-long-life and dendrite-free lithium-metal batteries. Adv Mater, 2021, 33: 2100943
Li Z, Zhou X Y, Guo X. High-performance lithium metal batteries with ultraconformal interfacial contacts of quasi-solid electrolyte to electrodes. Energy Storage Mater, 2020, 29: 149–155
Choudhury S, Mangal R, Agrawal A, et al. A highly reversible room-temperature lithium metal battery based on crosslinked hairy nanoparticles. Nat Commun, 2015, 6: 10101
Tu Z, Kambe Y, Lu Y, et al. Nanoporous polymer-ceramic composite electrolytes for lithium metal batteries. Adv Energy Mater, 2014, 4: 1300654
Byrne N, Efthimiadis J, MacFarlane D R, et al. The enhancement of lithium ion dissociation in polyelectrolyte gels on the addition of ceramic nano-fillers. J Mater Chem, 2004, 14: 127–133
Zou X, Lu Q, Zhong Y, et al. Flexible, flame-resistant, and dendrite-impermeable gel-polymer electrolyte for Li-O2/air batteries workable under hurdle conditions. Small, 2018, 14: 1801798
Choi C S, Lau J, Hur J, et al. Synthesis and properties of a photopatternable lithium-ion conducting solid electrolyte. Adv Mater, 2018, 30: 1703772
Zardalidis G, Pipertzis A, Mountrichas G, et al. Effect of polymer architecture on the ionic conductivity. Densely grafted poly(ethylene oxide) brushes doped with LiTF. Macromolecules, 2016, 49: 2679–2687
Chen G, Zhang F, Zhou Z, et al. A flexible dual-ion battery based on PVDF-HFP-modified gel polymer electrolyte with excellent cycling performance and superior rate capability. Adv Energy Mater, 2018, 8: 1801219
Duan J, Huang L, Wang T, et al. Shaping the contact between Li metal anode and solid-state electrolytes. Adv Funct Mater, 2020, 30: 1908701
Duan H, Yin Y X, Zeng X X, et al. In-situ plasticized polymer electrolyte with double-network for flexible solid-state lithium-metal batteries. Energy Storage Mater, 2018, 10: 85–91
Lin D, Liu W, Liu Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett, 2016, 16: 459–465
Cao J, Xie Y, Yang Y, et al. Achieving uniform Li plating/stripping at ultrahigh currents and capacities by optimizing 3D nucleation sites and Li2Se-enriched SEI. Adv Sci, 2022, 9: 2104689
Li Z, Xie H X, Zhang X Y, et al. In situ thermally polymerized solid composite electrolytes with a broad electrochemical window for all-solid-state lithium metal batteries. J Mater Chem A, 2020, 8: 3892–3900
Cho S J, Jung G Y, Kim S H, et al. Monolithic heterojunction quasi-solid-state battery electrolytes based on thermodynamically immiscible dual phases. Energy Environ Sci, 2019, 12: 559–565
Kim S H, Choi K H, Cho S J, et al. Printable solid-state lithium-ion batteries: A new route toward shape-conformable power sources with aesthetic versatility for flexible electronics. Nano Lett, 2015, 15: 5168–5177
Si Z, Li J, Ma L, et al. The ultrafast and continuous fabrication of a polydimethylsiloxane membrane by ultraviolet-induced polymerization. Angew Chem Int Ed, 2019, 58: 17175–17179
Jeon Y M, Kim S, Lee M, et al. Polymer-clay nanocomposite solidstate electrolyte with selective cation transport boosting and retarded lithium dendrite formation. Adv Energy Mater, 2020, 10: 2003114
Wen P, Lu P, Shi X, et al. Photopolymerized gel electrolyte with unprecedented room-temperature ionic conductivity for high-energy-density solid-state sodium metal batteries. Adv Energy Mater, 2021, 11: 2002930
Bruce P G, Vincent C A. Steady state current flow in solid binary electrolyte cells. J Electroanal Chem Interfacial Electrochem, 1987, 225: 1–17
Xu H, Wang A, Liu X, et al. A new fluorine-containing star-branched polymer as electrolyte for all-solid-state lithium-ion batteries. Polymer, 2018, 146: 249–255
Liu W, Lin D, Sun J, et al. Improved lithium ionic conductivity in composite polymer electrolytes with oxide-ion conducting nanowires. ACS Nano, 2016, 10: 11407–11413
He X, Ni Y, Hou Y, et al. A new insight into the ionic conduction mechanism of quasi-solid polymer electrolyte through multispectral characterizations. Angew Chem Int Ed, 2021, 60: 22672–22677
Nag A, Ali M A, Singh A, et al. N-Boronated polybenzimidazole for composite electrolyte design of highly ion conducting pseudo solidstate ion gel electrolytes with a high Li-transference number. J Mater Chem A, 2019, 7: 4459–4468
Han F, Zhu Y, He X, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv Energy Mater, 2016, 6: 1501590
Lee M, Hong J, Lee B, et al. Multi-electron redox phenazine for ready-to-charge organic batteries. Green Chem, 2017, 19: 2980–2985
Xie Y, Cao J, Wang X, et al. MOF-derived bifunctional Co0.85Se nanoparticles embedded in N-doped carbon nanosheet arrays as efficient sulfur hosts for lithium-sulfur batteries. Nano Lett, 2021, 21: 8579–8586
Li S, Zhang W, Liu J, et al. Maximizing catalytically active surface gallium for electrocatalysis of lithium polysulfides in lithium-sulfur batteries by silica@gallium core-shell particles. Appl Surf Sci, 2021, 563: 150381
Li Y, Xu B, Xu H, et al. Hybrid polymer/garnet electrolyte with a small interfacial resistance for lithium-ion batteries. Angew Chem Int Ed, 2017, 56: 753–756
Ye F, Zhang X, Liao K, et al. A smart lithiophilic polymer filler in gel polymer electrolyte enables stable and dendrite-free Li metal anode. J Mater Chem A, 2020, 8: 9733–9742
Chen S, Wen K, Fan J, et al. Progress and future prospects of highvoltage and high-safety electrolytes in advanced lithium batteries: From liquid to solid electrolytes. J Mater Chem A, 2018, 6: 11631–11663
Zhang Y, Sun X, Cao D, et al. Self-stabilized LiNi0.8Mn0.1Co0.1O2 in thiophosphate-based all-solid-state batteries through extra LiOH. Energy Storage Mater, 2021, 41: 505–514
Lee S, Lee K, Ku K, et al. Utilizing latent multi-redox activity of p-type organic cathode materials toward high energy density lithium-organic batteries. Adv Energy Mater, 2020, 10: 2001635
Liu W, Li J, Li W, et al. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat Commun, 2020, 11: 3629
Gu M, Fan L, Zhou J, et al. Regulating solvent molecule coordination with KPF6 for superstable graphite potassium anodes. ACS Nano, 2021, 15: 9167–9175
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the Recruitment Program of Global Experts, the Hundred-Talent Project of Fujian, and Fuzhou University.
Supporting Information
The supporting information is available online at https://tech.scichina.com and https://link.springer.com. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Rights and permissions
About this article
Cite this article
Zhang, W., Li, S., Zhang, Y. et al. A quasi-solid-state electrolyte with high ionic conductivity for stable lithium-ion batteries. Sci. China Technol. Sci. 65, 2369–2379 (2022). https://doi.org/10.1007/s11431-022-2075-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-022-2075-8