Abstract
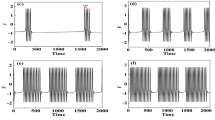
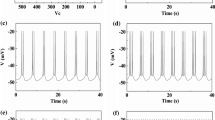
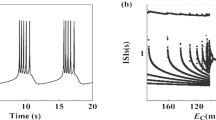
In the traditional viewpoint, inhibitory and excitatory effects always induce opposite responses. In the present study, the enhanced bursting activities induced by excitatory autapses, which are consistent with the recent experimental observations, and those induced by inhibitory autapses, which is a paradoxical phenomenon, were simulated using the Chay model. The same bifurcations and different ionic currents for the same responses were acquired with fast-slow variable dissection and current decomposition, respectively. As the inhibitory or excitatory autaptic conductance increased, the ending phase of the burst related to a homoclinic bifurcation of the fast subsystem changed to widen the burst duration to contain more spikes, which was induced by an elevated minimal potential (Vmin) of spiking of the fast subsystem. Larger inhibitory and excitatory autaptic conductances induced smaller and larger maximal potentials (Vmax) of spiking, respectively. During the downstroke, a weaker potassium current induced by the smaller Vmax played a dominant role for the inhibitory autapse, and the stronger potassium current induced by the larger Vmax became weaker due to the opposite autaptic current of the excitatory autapse, which induced the Vmin elevated. The results present the nonlinear and biophysical mechanisms of the same responses to opposite effects, which extends nonlinear dynamics knowledge and provides potential modulation measures for the nervous system.
Similar content being viewed by others
References
Braun H A, Wissing H, Schäfer K, et al. Oscillation and noise determine signal transduction in shark multimodal sensory cells. Nature, 1994, 367: 270–273
Elbert T, Ray W J, Kowalik Z J, et al. Chaos and physiology: Deterministic chaos in excitable cell assemblies. Physiol Rev, 1994, 74: 1–47
Glass L. Synchronization and rhythmic processes in physiology. Nature, 2001, 410: 277–284
Zhang X J, Gu H G, Guan L N. Stochastic dynamics of conduction failure of action potential along nerve fiber with Hopf bifurcation. Sci China Tech Sci, 2019, 62: 1502–1511
Rinzel J, Lee Y S. Dissection of a model for neuronal parabolic bursting. J Math Biol, 1987, 25: 653–675
Izhikevich E M, Desai N S, Walcott E C, et al. Bursts as a unit of neural information: Selective communication via resonance. Trends Neurosciences, 2003, 26: 161–167
Jia B, Gu H, Xue L. A basic bifurcation structure from bursting to spiking of injured nerve fibers in a two-dimensional parameter space. Cogn Neurodyn, 2017, 11: 189–200
Yang Y, Cui Y, Sang K, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature, 2018, 554: 317–322
Izhikevich E M. Neural excitability, spiking and bursting. Int J Bifurcation Chaos, 2000, 10: 1171–1266
Li Y, Gu H. The distinct stochastic and deterministic dynamics between period-adding and period-doubling bifurcations of neural bursting patterns. Nonlinear Dyn, 2017, 87: 2541–2562
Saada R, Miller N, Hurwitz I, et al. Autaptic excitation elicits persistent activity and a plateau potential in a neuron of known behavioral function. Curr Biol, 2009, 19: 479–484
Guo D, Chen M, Perc M, et al. Firing regulation of fast-spiking interneurons by autaptic inhibition. EPL, 2016, 114: 30001
Mondal A, Upadhyay R K, Ma J, et al. Bifurcation analysis and diverse firing activities of a modified excitable neuron model. Cogn Neurodyn, 2019, 13: 393–407
Goldwyn J H, Slabe B R, Travers J B, et al. Gain control with A-type potassium current: IA as a switch between divisive and subtractive inhibition. PLoS Comput Biol, 2018, 14: e1006292
Hörning M, Nakahata M, Linke P, et al. Dynamic mechano-regulation of myoblast cells on supramolecular hydrogels cross-linked by reversible host-guest interactions. Sci Rep, 2017, 7: 7660
Zhao Z, Li L, Gu H. Excitatory autapse induces different cases of reduced neuronal firing activities near Hopf bifurcation. Commun Nonlinear Sci Numer Simul, 2020, 85: 105250
Sun X, Si H. Population rate coding in recurrent neuronal networks consisting of neurons with mixed excitatory-inhibitory synapses. Nonlinear Dyn, 2020, 100: 2673–2686
Sun X, Li G. Synchronization transitions induced by partial time delay in a excitatory-inhibitory coupled neuronal network. Nonlinear Dyn, 2017, 89: 2509–2520
Perkel D H, Schulman J H, Bullock T H, et al. Pacemaker neurons: Effects of regularly spaced synaptic input. Science, 1964, 145: 61–63
Satterlie R A. Reciprocal inhibition and postinhibitory rebound produce reverberation in a locomotor pattern generator. Science, 1985, 229: 402–404
Winograd M, Destexhe A, Sanchez-Vives M V. Hyperpolarization-activated graded persistent activity in the prefrontal cortex. Proc Natl Acad Sci USA, 2008, 105: 7298–7303
Elgueta C, Kohler J, Bartos M. Persistent discharges in dentate gyrus perisoma-inhibiting interneurons require hyperpolarization-activated cyclic nucleotide-gated channel activation. J Neuroscience, 2015, 35: 4131–4139
Zhao Z, Li L, Gu H. Dynamical mechanism of hyperpolarization-activated non-specific cation current induced resonance and spike-timing precision in a neuronal model. Front Cell Neurosci, 2018, 12
Zhao Z, Li L, Gu H, et al. Different dynamics of repetitive neural spiking induced by inhibitory and excitatory autapses near subcritical Hopf bifurcation. Nonlinear Dyn, 2020, 99: 1129–1154
Guan L, Jia B, Gu H. A novel threshold across which the negative stimulation evokes action potential near a saddle-node bifurcation in a neuronal model with IH current. Int J Bifurcation Chaos, 2019, 29: 1950198
Dodla R, Rinzel J. Enhanced neuronal response induced by fast inhibition. Phys Rev E, 2006, 73: 010903
Dodla R, Svirskis G, Rinzel J. Well-timed, brief inhibition can promote spiking: Postinhibitory facilitation. J NeuroPhysiol, 2006, 95: 2664–2677
Beiderbeck B, Myoga M H, Müller N I C, et al. Precisely timed inhibition facilitates action potential firing for spatial coding in the auditory brainstem. Nat Commun, 2018, 9: 1771
Wu F, Gu H, Li Y. Inhibitory electromagnetic induction current induces enhancement instead of reduction of neural bursting activities. Commun Nonlinear Sci Numer Simul, 2019, 79: 104924
Wu F, Gu H. Bifurcations of negative responses to positive feedback current mediated by memristor in a neuron model with bursting patterns. Int J Bifurcation Chaos, 2020, 30: 2030009
Wang X J, Rinzel J. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Computation, 1992, 4: 84–97
Jalil S, Belykh I, Shilnikov A. Fast reciprocal inhibition can synchronize bursting neurons. Phys Rev E, 2010, 81: 045201
Jia B, Wu Y, He D, et al. Dynamics of transitions from anti-phase to multiple in-phase synchronizations in inhibitory coupled bursting neurons. Nonlinear Dyn, 2018, 93: 1599–1618
Uzuntarla M, Torres J J, Calim A, et al. Synchronization-induced spike termination in networks of bistable neurons. Neural Networks, 2019, 110: 131–140
Cobb S R, Halasy K, Vida I, et al. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience, 1997, 79: 629–648
Bacci A, Huguenard J R. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron, 2006, 49: 119–130
Van Der Loos H, Glaser E M. Autapses in neocortex cerebri: Synapses between a pyramidal cell’s axon and its own dendrites. Brain Res, 1972, 48: 355–360
Bekkers J M, Stevens C F. Excitatory and inhibitory autaptic currents in isolated hippocampal neurons maintained in cell culture. Proc Natl Acad Sci USA, 1991, 88: 7834–7838
Jiang M, Zhu J, Liu Y P, et al. Enhancement of asynchronous release from fast-spiking interneuron in human and rat epileptic neocortex. PLoS Biol, 2012, 10: e1001324
Yin L, Zheng R, Ke W, et al. Autapses enhance bursting and coincidence detection in neocortical pyramidal cells. Nat Commun, 2018, 9: 4890
Ke W, He Q, Shu Y. Functional self-excitatory autapses (auto-synapses) on neocortical pyramidal cells. Neurosci Bull, 2019, 35: 1106–1109
Bacci A, Huguenard J R, Prince D A. Functional autaptic neurotransmission in fast-spiking interneurons: A novel form of feedback inhibition in the neocortex. J Neurosci, 2003, 23: 859–866
Li Y, Schmid G, Hänggi P, et al. Spontaneous spiking in an autaptic Hodgkin-Huxley setup. Phys Rev E, 2010, 82: 061907
Hashemi M, Valizadeh A, Azizi Y. Effect of duration of synaptic activity on spike rate of a Hodgkin-Huxley neuron with delayed feedback. Phys Rev E, 2012, 85: 021917
Wang H, Ma J, Chen Y, et al. Effect of an autapse on the firing pattern transition in a bursting neuron. Commun Nonlinear Sci Numer Simul, 2014, 19: 3242–3254
Wang H, Wang L, Chen Y, et al. Effect of autaptic activity on the response of a Hodgkin-Huxley neuron. Chaos, 2014, 24: 033122
Song X L, Wang C N, Ma J, et al. Transition of electric activity of neurons induced by chemical and electric autapses. Sci China Tech Sci, 2015, 58: 1007–1014
Guo D, Wu S, Chen M, et al. Regulation of irregular neuronal firing by autaptic transmission. Sci Rep, 2016, 6: 26096
Song X, Wang H, Chen Y. Autapse-induced firing patterns transitions in the MorrisCLecar neuron model. Nonlinear Dyn, 2019, 96: 2341–2350
Qin H X, Ma J, Jin W Y, et al. Dynamics of electric activities in neuron and neurons of network induced by autapses. Sci China Tech Sci, 2014, 57: 936–946
Qin H, Wu Y, Wang C, et al. Emitting waves from defects in network with autapses. Commun Nonlinear Sci Numer Simul, 2015, 23: 164–174
Yilmaz E, Ozer M, Baysal V, et al. Autapse-induced multiple coherence resonance in single neurons and neuronal networks. Sci Rep, 2016, 6: 30914
Ge M, Xu Y, Zhang Z, et al. Autaptic modulation-induced neuronal electrical activities and wave propagation on network under electromagnetic induction. Eur Phys J Spec Top, 2018, 227: 799–809
Tikidji-Hamburyan R A, Martínez J J, White J A, et al. Resonant interneurons can increase robustness of gamma oscillations. J Neurosci, 2015, 35: 15682–15695
Li Y Y, Gu H G, Ding X L. Bifurcations of enhanced neuronal bursting activities induced by the negative current mediated by inhibitory autapse. Nonlinear Dyn, 2019, 97: 2091–2105
Ding X L, Jia B, Li Y Y. Explanation to negative feedback inducedenhancement of neural electronic activities with phase response curve (in Chinese). Acta Phys Sin, 2019, 68: 180502
Yao C, He Z, Nakano T, et al. Inhibitory-autapse-enhanced signal transmission in neural networks. Nonlinear Dyn, 2019, 97: 1425–1437
Prescott S A, Koninck Y D, Sejnowski T J. Biophysical basis for three distinct dynamical mechanisms of action potential initiation. PLoS Comput Biol, 2008, 4: e1000198
Drion G, OLeary T, Marder E. Ion channel degeneracy enables robust and tunable neuronal firing rates. Proc Natl Acad Sci USA, 2015, 112: E5361–E5370
Chay T R. Chaos in a three-variable model of an excitable cell. Physica D-Nonlinear Phenomena, 1985, 16: 233–242
Fan Y S, Chay T R. Generation of periodic and chaotic bursting in an excitable cell model. Biol Cybern, 1994, 71: 417–431
Jalil S, Belykh I, Shilnikov A. Spikes matter for phase-locked bursting in inhibitory neurons. Phys Rev E, 2012, 85: 036214
Cymbalyuk G S, Gaudry Q, Masino M A, et al. Bursting in leech heart interneurons: Cell-autonomous and network-based mechanisms. J Neurosci, 2002, 22: 10580–10592
Ermentrout B. Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. Philadelphia: SIAM, 2002
Zhou X, Xu Y, Wang G, et al. Ionic channel blockage in stochastic HodgkinCHuxley neuronal model driven by multiple oscillatory signals. Cogn Neurodyn, 2020, 14: 569–578
Wang Z, Shi X. Electric activities of time-delay memristive neuron disturbed by Gaussian white noise. Cogn Neurodyn, 2020, 14: 115–124
Cao B, Guan L N, Gu H G. Bifurcation mechanism of not increase but decrease of spike number within a neural burst induced by excitatory effect (in Chinese). Acta Phys Sin, 2018, 67: 240502
Zhao Z, Li L, Gu H. Different dynamical behaviors induced by slow excitatory feedback for type II and III excitabilities. Sci Rep, 2020, 10: 3646
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the National Natural Science Foundation of China (Grant Nos. 11762001, 11402055 and 11872276), the Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (Grant No. NJYT-20-A09), and the Program for Excellent Young Talents in Colleges and Universities of Anhui Province of China (Grant No. gxyqZD2020077).
Rights and permissions
About this article
Cite this article
Li, Y., Gu, H., Jia, B. et al. The nonlinear mechanism for the same responses of neuronal bursting to opposite self-feedback modulations of autapse. Sci. China Technol. Sci. 64, 1459–1471 (2021). https://doi.org/10.1007/s11431-020-1753-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-020-1753-y