Abstract
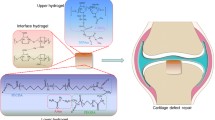
Osteochondral defects are most commonly characterized by damages to both cartilage and underlying subchondral bone tissues, thus developing bi-layered scaffold that can concurrently regenerate these two specific lineages becomes challenge. In this study, the highly biomimetic bi-layered scaffolds were successfully prepared using human-like-collagen (HLC), hyaluronan (HA) and nano hydroxyapatite (HAP) particles, combined with “liquid phase synthesis” technology, freeze-drying and chemical cross-linking techniques, which was simulated the composition of natural extracellular matrix to repair osteochondral defects. This novel bilayer osteochondral graft had a seamlessly integrated layer structure, suitable pore size, high levels of porosity, and excellent mechanical properties. In vitro cell experiments of the bilayer scaffold indicated that the scaffold could promote the proliferation and adhesion of human bone marrow mesenchymal stem cells. In vivo osteochondral defects and micro-CT experiment revealed that bilayer scaffolds showed complete closure of the defect. Histology confirmed collagen and glycosaminoglycans were deposited in the new matrix of hyaline cartilage and bone in the bilayer scaffold group. Therefore, the developed bionic bilayer scaffold enhanced the regeneration of hyaline cartilage through subchondral bone formation and lateral host-tissue integration. In conclusion, this bilayer scaffold based on HLC could be used as the desired strategy for osteochondral defects regeneration.
Similar content being viewed by others
References
Martin I, Miot S, Barbero A, et al. Osteochondral tissue engineering. J BioMech, 2007, 40: 750–765
Wu Y, Zhu S, Wu C, et al. A bi-lineage conducive scaffold for osteochondral defect regeneration. Adv Funct Mater, 2014, 24: 4473–4483
Yuan X L, Meng H Y, Wang Y C, et al. Bone-cartilage interface crosstalk in osteoarthritis: potential pathways and future therapeutic strategies. Osteoarthritis Cartilage, 2014, 22: 1077–1089
Zhang Y, Yu J, Ren K, et al. Thermosensitive hydrogels as scaffolds for cartilage tissue rngineering. Biomacromolecules, 2019, 20: 1478–1492
Huey D J, Hu J C, Athanasiou K A. Unlike bone, cartilage regeneration remains elusive. Science, 2012, 338: 917–921
Yan L P, Silva-Correia J, Oliveira M B, et al. Bilayered silk/silknanoCaP scaffolds for osteochondral tissue engineering: In vitro and in vivo assessment of biological performance. Acta Biomater, 2015, 12: 227–241
Kang H, Zeng Y, Varghese S. Functionally graded multilayer scaffolds for in vivo osteochondral tissue engineering. Acta Biomater, 2018, 78: 365–377
Xia D, Liu Y, Wang S, et al. In vitro and in vivo investigation on biodegradable Mg-Li-Ca alloys for bone implant application. Sci China Mater, 2019, 62: 256–272
Xu Q, Liang J, Xue H, et al. Novel injectable and self-setting composite materials for bone defect repair. Sci China Mater, 2020, 63: 876–887
Levingstone T J, Thompson E, Matsiko A, et al. Multi-layered collagen-based scaffolds for osteochondral defect repair in rabbits. Acta Biomater, 2016, 32: 149–160
Wakitani S, Goto T, Young R G, et al. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng, 1998, 4: 429–444
Lahm A, Kreuz P C, Oberst M, et al. Subchondral and trabecular bone remodeling in canine experimental osteoarthritis. Arch Orthop Trauma Surg, 2006, 126: 582–587
Mrosek E H, Lahm A, Erggelet C, et al. Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine model. Osteoarthritis Cartilage, 2006, 14: 171–178
O’Shea T M, Miao X. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B-Rev, 2008, 14: 447–464
Oliveira J M, Rodrigues M T, Silva S S, et al. Novel hydroxyapatite/chitosan bilayered scaffold for osteochondral tissue-engineering applications: Scaffold design and its performance when seeded with goat bone marrow stromal cells. Biomaterials, 2006, 27: 6123–6137
Reyes R, Delgado A, Sánchez E, et al. Repair of an osteochondral defect by sustained delivery of BMP-2 or TGF²1 from a bilayered alginate-PLGA scaffold. J Tissue Eng Regen Med, 2012
Guo X, Park H, Liu G, et al. In vitro generation of an osteochondral construct using injectable hydrogel composites encapsulating rabbit marrow mesenchymal stem cells. Biomaterials, 2009, 30: 2741–2752
Kon E, Delcogliano M, Filardo G, et al. Novel nano-composite multilayered biomaterial for osteochondral regeneration. Am J Sports Med, 2011, 39: 1180–1190
Chen J, Chen H, Li P, et al. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials, 2011, 32: 4793–4805
Moutos F T, Freed L E, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater, 2007, 6: 162–167
Yang P J, Temenoff J S. Engineering Orthopedic Tissue Interfaces. Tissue Eng Part B-Rev, 2009, 15: 127–141
Gomes S, Leonor I B, Mano J F, et al. Natural and genetically engineered proteins for tissue engineering. Prog Polym Sci, 2012, 37: 1–17
Huang X, Jia J, Wang Z, et al. A novel chitosan-based sponge coated with self-assembled thrombin/tannic acid multilayer films as a hemostatic dressing. Chin J Polym Sci, 2015, 33: 284–290
Geng C, Hu X, Yang G, et al. Mechanically reinforced chitosan/cellulose nanocrystals composites with good transparency and biocompatibility. Chin J Polym Sci, 2015, 33: 61–69
Hu J, Cai X, Mo S, et al. Fabrication and characterization of chitosansilk fibroin/hydroxyapatite composites via in situ precipitation for bone tissue engineering. Chin J Polym Sci, 2015, 33: 1661–1671
Xu W K, Tang J Y, Yuan Z, et al. Accelerated cutaneous wound healing using an injectable teicoplanin-loaded PLGA-PEG-PLGA thermogel dressing. Chin J Polym Sci, 2019, 37: 548–559
Park S H, Song T, Bae T S, et al. Comparative analysis of collagens extracted from different animal sources for application of cartilage tissue engineering. Int J Precis Eng Manuf, 2012, 13: 2059–2066
Zhang L, Liu J, Zheng X, et al. Pullulan dialdehyde crosslinked gelatin hydrogels with high strength for biomedical applications. Carbohydrate Polyms, 2019, 216: 45–53
Ramshaw J A M. Biomedical applications of collagens. J Biomed Mater Res, 2016, 104: 665–675
Sionkowska A, Kozlowska J. Fish scales as a biocomposite of collagen and calcium salts. KEM, 2013, 587: 185–190
Fan D D, Luo Y, Mi Y, et al. Characteristics of fed-batch cultures of recombinant Escherichia coli containing human-like collagen cDNA at different specific growth rates. Biotechnol Lett, 2005, 27: 865–870
Zhang J, Ma X, Fan D, et al. Synthesis and characterization of hyaluronic acid/human-like collagen hydrogels. Mater Sci Eng-C, 2014, 43: 547–554
Li X, Xue W, Liu Y, et al. Novel multifunctional PB and PBH hydrogels as soft filler for tissue engineering. J Mater Chem B, 2015, 3: 4742–4755
Jiang X, Wang Y, Fan D, et al. A novel human-like collagen hemostatic sponge with uniform morphology, good biodegradability and biocompatibility. J Biomater Appl, 2017, 31: 1099–1107
Zhu C, Fan D, Wang Y. Human-like collagen/hyaluronic acid 3D scaffolds for vascular tissue engineering. Mater Sci Eng-C, 2014, 34: 393–401
Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules, 2011, 12: 1387–1408
Yang J, Zhang Y S, Yue K, et al. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater, 2017, 57: 1–25
Matsiko A, Levingstone T J, O’Brien F J, et al. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J Mech Behav BioMed Mater, 2012, 11: 41–52
Yang Q, Peng J, Guo Q, et al. A cartilage ECM-derived 3-D porous acellular matrix scaffold for in vivo cartilage tissue engineering with PKH26-labeled chondrogenic bone marrow-derived mesenchymal stem cells. Biomaterials, 2008, 29: 2378–2387
Zheng X F, Lu S B, Zhang W G, et al. Mesenchymal stem cells on a decellularized cartilage matrix for cartilage tissue engineering. Biotechnol Bioproc E, 2011, 16: 593–602
Zheng X, Yang F, Wang S, et al. Fabrication and cell affinity of biomimetic structured PLGA/articular cartilage ECM composite scaffold. J Mater Sci-Mater Med, 2011, 22: 693–704
Yang F, Qu X, Cui W, et al. Manufacturing and morphology structure of polylactide-type microtubules orientation-structured scaffolds. Biomaterials, 2006, 27: 4923–4933
Harley B A, Lynn A K, Wissner-Gross Z, et al. Design of a multiphase osteochondral scaffold III: Fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res, 2009, 9999A: NA
McNamara S L, Rnjak-Kovacina J, Schmidt D F, et al. Silk as a biocohesive sacrificial binder in the fabrication of hydroxyapatite load bearing scaffolds. Biomaterials, 2014, 35: 6941–6953
Lu S, Lam J, Trachtenberg J E, et al. Dual growth factor delivery from bilayered, biodegradable hydrogel composites for spatially-guided osteochondral tissue repair. Biomaterials, 2014, 35: 8829–8839
Radhakrishnan J, Manigandan A, Chinnaswamy P, et al. Gradient nano-engineered in situ forming composite hydrogel for osteochondral regeneration. Biomaterials, 2018, 162: 82–98
Sophia Fox A J, Bedi A, Rodeo S A. The basic science of articular cartilage: Structure, composition, and function. Sports Health, 2009, 1: 461–468
Zhang Q, Lu H, Kawazoe N, et al. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater, 2014, 10: 2005–2013
Huang J, Xia X, Zou Q, et al. The long-term behaviors and differences in bone reconstruction of three polymer-based scaffolds with different degradability. J Mater Chem B, 2019, 7: 7690–7703
Ribeiro V P, Pina S, Costa J B, et al. Enzymatically cross-linked silk fibroin-based hierarchical scaffolds for osteochondral regeneration. ACS Appl Mater Interfaces, 2019, 11: 3781–3799
Drury J L, Mooney D J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials, 2003, 24: 4337–4351
Madry H, van Dijk C N, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc, 2010, 18: 419–433
Song X, Zhu C, Fan D, et al. A novel human-like collagen hydrogel scaffold with porous structure and sponge-like properties. Polymers, 2017, 9: 638
Naseri N, Poirier J M, Girandon L, et al. 3-Dimensional porous nanocomposite scaffolds based on cellulose nanofibers for cartilage tissue engineering: Tailoring of porosity and mechanical performance. RSC Adv, 2016, 6: 5999–6007
Wu X, Liu Y, Li X, et al. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater, 2010, 6: 1167–1177
Murphy C M, Schindeler A, Gleeson J P, et al. A collagen-hydroxyapatite scaffold allows for binding and co-delivery of recombinant bone morphogenetic proteins and bisphosphonates. Acta Biomater, 2014, 10: 2250–2258
Gleeson J P, Plunkett N A, O’Brien F J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. eCM, 2010, 20: 218–230
Vines J B, Lim D J, Anderson J M, et al. Hydroxyapatite nanoparticle reinforced peptide amphiphile nanomatrix enhances the osteogenic differentiation of mesenchymal stem cells by compositional ratios. Acta Biomater, 2012, 8: 4053–4063
Author information
Authors and Affiliations
Corresponding authors
Additional information
This work was supported by the National Key R&D Program of China (Grant No. 2019YFA0905200), National Natural Science Foundation of China (Grant Nos. 21838009, 21878247, and 21676214) and the Shaanxi Key Laboratory of Degradable Biomedical Materials Program (Grant No. 17JS124).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, K., Liu, Y., Duan, Z. et al. A biomimetic bi-layered tissue engineering scaffolds for osteochondral defects repair. Sci. China Technol. Sci. 64, 793–805 (2021). https://doi.org/10.1007/s11431-020-1597-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-020-1597-4