Abstract
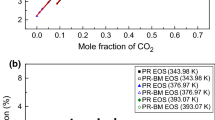
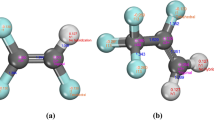
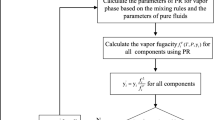
An accurate knowledge about phase behaviors of CH4, CO2 and their binary mixture is crucial in fields of natural gas liquefaction and refrigeration applications. In this work, two all-atom force fields of TraPPE-EH and EMP2 were used for the components CH4 and CO2, respectively. Then the vapor-liquid equilibria (VLE) of CH4, CO2 and their binary system were calculated via the NVT- and NpT Gibbs Ensemble Monte Carlo Simulations. Meanwhile the traditional method using Equation of State (EoS) to correlate the VLE properties was also investigated. The EoSs considered in this work were three classic cubic RK, SRK, PR and another advanced molecular-based PC-SAFT equations. For pure components, both molecular simulations and the PC-SAFT EoS could obtain satisfactory predictions for all the saturated properties. However, the saturated liquid densities calculated by the cubic EoSs were not so good. It was also observed that the TraPPE-EH force field had a good representation for CH4 molecule, while the EMP2 force field was not enough accurate to represent CO2 molecules. For the mixture CH4 + CO2, SRK and PR showed the best predictions for the saturated pressure-component property, while good results were also obtained via molecular simulations and PC-SAFT EoS. It was suggested that special combining rules or binary interaction parameters were important to obtain enough accurate prediction of the mixed phase behavior. Compared with the cubic EoS, the PC-SAFT and molecular simulation method showed better adaptabilities for both the pure and mixture systems. Besides, the accurate molecular parameters used in the PC-SAFT and molecular simulations could bring about direct and deep understanding about the molecular characteristics.
Similar content being viewed by others
References
Kosseim A. New development in gas purification for LNG plants. In: Tenth International Conference & Exhibition on Liquefied Natural Gas, 1992
Cao W S. Study on natural gas purification and liquefaction process of small scale LNG project. Dissertation of Doctor Degree. Shanghai: Shanghai Jiaotong University, 2008
Oldenburg C M, Pruess K, Benson S M. Process modeling of CO2 injection into natural gas reservoirs for carbon sequestration and enhanced gas recovery. Energy Fuels, 2001, 15: 293–298
Class H, Ebigbo A, Helmig R, et al. A benchmark study on problems related to CO2 storage in geologic formations. Comput Geosci, 2009, 13: 409–434
Han C, Zhang P, Ye T H, et al. Numerical study of methane/air jet flame in vitiated co-flow using tabulated detailed chemistry. Sci China Tech Sci, 2014, 57: 1750–1760
Yu J, Meng H. A numerical study of counterflow diffusion flames of methane/air at various pressures. Sci China Tech Sci, 2014, 57: 615–624
Kvenvolden K A. Gas hydrates-geological perspective and global change. Rev Geophys, 1993, 31: 173–187
Deusner C, Bigalke N, Kossel E, et al. Methane production from gas hydrate deposits through injection of supercritical CO2. Energies, 2012, 5: 2112–2140
Kanoğlu M. Exergy analysis of multistage cascade refrigeration cycle used for natural gas liquefaction. Int J Energ Res, 2002, 26: 763–774
Lim W, Choi K, Moon I. Current status and perspectives of liquefied natural gas (LNG) plant design. Ind Eng Chem Res, 2013, 52: 3065–3088
Redlich O, Kwong J N S. On the thermodynamics of solutions. V. An equation of state. Fugacities of gaseous solutions. Chem Rev, 1949, 44: 233–244
Soave, G. Equilibrium constants from a modified Redlich-Kwong equation of state. Chem Eng Sci, 1972, 27: 1197–1203
Peng D Y, Robinson D B. A new two-constant equation of state. Ind Eng Chem Fundam, 1976, 15: 59–64
Chapman W G, Gubbins K E, Jackson G, et al. SAFT: Equation-of-state solution model for associating fluids. Fluid Phase Equilib, 1989, 52: 31–38
Huang S H, Radosz M. Equation of state for small, large, polydisperse, and associating molecules. Ind Eng Chem Res, 1990, 29: 2284–2294
Gross J, Sadowski G. Perturbed-chain SAFT: An equation of state based on a perturbation theory for chain molecules. Ind Eng Chem Res, 2001, 40: 1244–1260
Panagiotopoulos A Z. Direct determination of fluid phase equilibria by simulation in the Gibbs ensemble: A review. Mol Sim, 1992, 9: 1–23
Skvorova M, Smith W R. Molecular-level simulation of bubble and dew points of fluid mixtures and application to refrigerant cycle design. Int J Refrig, 2014, 42: 1–7
Smith W R, Figueroa-Gerstenmaier S, Skvorova M. Molecular simulation for thermodynamic properties and process modeling of refrigerants. J Chem Engin Data, 2014, 59: 3258–3271
Figueroa-Gerstenmaier S, Lísal M, Nezbeda I, et al. Prediction of isoenthalps, Joule-Thomson Coefficients and Joule-Thomson inversion curves of refrigerants by molecular simulation Fluid Phase Equilibr, 2014, 375: 143–151
Vinš V, Hrubý J. Solubility of nitrogen in one-component refrigerants: Prediction by PC-SAFT EoS and a correlation of Henry’s law constants. Int J Refrig, 2011, 34: 2109–2117
Marcelino Neto M A, Barbosa Jr J R. Prediction of refrigerant-lubricant viscosity using the general PC-SAFT friction theory. Int J Refrig, 2014, 45: 92–99
Polishuk I, Assor E, Cohen N, et al. Implementation of PC-SAFT and SAFT+ Cubic for modeling thermodynamic properties of haloalkanes. II. 7 Haloethanes and their mixtures. Int J Refrig, 2013, 36: 980–991
Liu Y, Panagiotopoulos A Z, Debenedetti P G. Monte carlo simulations of high-pressure phase equilibria of CO2-H2O mixtures. J Phys Chem B, 2011, 115: 6629–6635
Lin W, Yang Q, Zhong C. Molecular simulation of vapor-liquid equilibria of toxic gases. Fluid Phase Equilibr, 2004, 220: 1–6
Do H, Wheatley R J, Hirst J D. Microscopic structure of liquid 1-1-1-2-tetrafluoroethane (R134a) from Monte Carlo simulation. Phys Chem Chem Phys, 2010, 12: 13266–13272
Yamakov V, Wolf D, Phillpot S R, et al. Deformation-mechanism map for nanocrystalline metals by molecular-dynamics simulation. Nat Mater, 2003, 3: 43–47
Vrabec J, Fischer J. Vapor-liquid equilibria of binary mixtures containing methane, ethane, and carbon dioxide from molecular simulation. Int J Thermophys, 1996, 17: 889–908
Liu A, Beck T L. Vapor-liquid equilibria of binary and ternary mixtures containing methane, ethane, and carbon dioxide from Gibbs ensemble simulations. J Phys Chem B, 1998, 102: 7627–7631
Peguin R P S, Kamath G, Potoff J J, et al. All-atom force field for the prediction of vapor-liquid equilibria and interfacial properties of HFA134a. J Phys Chem B, 2008, 113: 178–187
Raabe G, Maginn E J. A force field for 3, 3, 3-fluoro-1-propenes, including HFO-1234yf. J Phys Chem B, 2010, 114: 10133–10142
Raabe G. Molecular simulation studies on the vapor-liquid phase equilibria of binary mixtures of R-1234yf and R-1234ze (E) with R-32 and CO2. J Chem Engin Data, 2013, 58: 1867–1873
Chen B, Siepmann J I. Transferable potentials for phase equilibria. 3. Explicit-hydrogen description of normal alkanes. J Phys Chem B, 1999, 103: 5370–5379
Harris J G, Yung K H. Carbon dioxide’s liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model. J Phys Chem, 1995, 99: 12021–12024
Daridon J L, Saint-Guirons H, La Courette B, et al. A generalized process for phase equilibrium calculation with cubic equations of state. Int J Thermophys, 1993, 14: 1101–1108
Grandjean L, de Hemptinne J C, Lugo R. Application of GC-PPC-SAFT EoS to ammonia and its mixtures. Fluid Phase Equilibr, 2014, 367: 159–172
NguyenHuynh D, Passarello J P, Tobaly P, et al. Application of GC-SAFT EOS to polar systems using a segment approach. Fluid Phase Equilibr, 2008, 264: 62–75
Gross J, Sadowski G. Application of the perturbed-chain SAFT equation of state to associating systems. Ind Eng Chem Res, 2002, 41: 5510–5515
Diamantonis N I, Economou I G. Evaluation of statistical associating fluid theory (SAFT) and perturbed Chain-SAFT equations of state for the calculation of thermodynamic derivative properties of fluids related to carbon capture and sequestration. Energy Fuels, 2011, 25: 3334–3343
Cornell W D, Cieplak P, Bayly C I, et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc, 1995, 117: 5179–5197
MacKerell A D, Bashford D, Bellott M, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J Phys Chem B, 1998, 102: 3586–3616
Jorgensen W L, Maxwell D S, Tirado-Rives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc, 1996, 118: 11225–11236
Panagiotopoulos A Z. Direct determination of phase coexistence properties of fluids by Monte Carlo simulation in a new ensemble. Mol Phys, 1987, 61: 813–826
Kofke D A. Direct evaluation of phase coexistence by molecular simulation via integration along the saturation line. J Chem Phys, 1993, 98: 4149–4162
Vrabec J, Stoll J, Hasse H. A set of molecular models for symmetric quadrupolar fluids. J Phys Chem B, 2001, 105: 12126–12133
Potoff J J, Panagiotopoulos A Z. Critical point and phase behavior of the pure fluid and a Lennard-Jones mixture. J Chem Phys, 1998, 109: 10914–10920
Allen M P, Tildesley D J. Computer Simulation of Liquids. Oxford: Clarendon, 1987
Kleinrahm R, Wagner W. Measurement and correlation of the equilibrium liquid and vapour densities and the vapour pressure along the coexistence curve of methane. J Chem Thermodyn, 1986, 18: 739–760
Lemmon E W, Huber M L, McLinden M O. 2007, NIST Reference Fluid Thermodynamic and Transport Properties-REFPROP, NIST Standard Reference Database 23-Version 8.0
Duschek W, Kleinrahm R, Wagner W. Measurement and correlation of the (pressure, density, temperature) relation of carbon dioxide II. Saturated-liquid and saturated-vapour densities and the vapour pressure along the entire coexistence curve. J Chem Thermodyn, 1990, 22: 841–864
Rowlinson J S, Swinton F L. Liquids and Liquid Mixtures. 3rd ed. London: Butterworth, 1982
Rowlinson J S, Widom B. Molecular Theory of Capillarity. Oxford: Clarendon Press, 1982
Davalos J, Anderson W R, Phelps R E, et al. Liquid-vapor equilibria at 250.00. deg. K for systems containing methane, ethane, and carbon dioxide. J Chem Eng Data, 1976, 21: 81–84
Wei M S W, Brown T S, Kidnay A J, et al. Vapor+ liquid equilibria for the ternary system methane+ ethane+ carbon dioxide at 230 K and its constituent binaries at temperatures from 207 to 270 K. J Chem Eng Data, 1995, 40: 726–731
Vrabec J, Stoll J, Hasse H. Molecular models of unlike interactions in fluid mixtures. Mol Sim, 2005, 31: 215–221
Schnabel T, Vrabec J, Hasse H. Unlike Lennard-Jones parameters for vapor-liquid equilibria. Mol Liq, 2007, 135: 170–178
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Z., Gong, M., Zhou, Y. et al. Vapor-liquid equilibria of CH4, CO2 and their binary system CH4 + CO2: A comparison between the molecular simulation and equation of state. Sci. China Technol. Sci. 58, 650–658 (2015). https://doi.org/10.1007/s11431-015-5785-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11431-015-5785-4