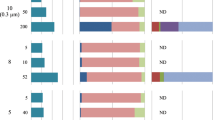
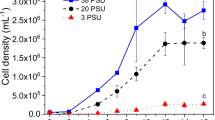
Abstract
Membrane lipids are thought to be a crucial part of the homeoviscous adaptation of archaea to extreme conditions. This article reviews the recent lipidomic studies of physiological membrane adaptations of archaea, assesses the biomolecular basis of an organic paleothermometer, TEX86, and contemplates the future directions of archaeal lipidomics. The studies of extremophilic archaea have revealed that at least three different molecular mechanisms are involved in membrane adaptation of archaea: (1) regulation of the number of cyclopentane rings of caldarchaeol, (2) alteration of the diether-to-tetraether lipid ratio, and (3) variation of the proportion of saturated and unsaturated lipids. However, most of the studies have focused on a limited number of archaeal ether-linked lipids, such as glycerol dialkyl glycerol tetraethers (GDGTs), which only represent a fraction of the entire lipidome. Environmental factors such as growth temperature and pH have been most frequently reported, but biotic factors, including growth phases, nutrition, and enzymatic activities affecting the membrane lipid composition are often overlooked. Membrane lipids of mesophilic ammonia-oxidizing marine Thaumarchaeota have been applied in the reconstruction of past sea surface temperatures. However, recent culture-based physiological studies have demonstrated that non-thermal biotic factors, including dissolved oxygen, ammonia oxidation rate and the growth rate, are the main drivers of GDGT cyclization in Nitrosopumilus maritimus. Moreover, other related strains or ecotypes exhibit a markedly different set of stress adaptations. A trend is now developing to examine the whole lipid profile (lipidome) for studies of archaeal physiology and biochemistry related to lipid biosynthesis (lipidomics) to gain a better understanding of the biological mechanisms underpinning the applications of membrane lipid-based proxies in biogeochemical or ecological research.
Similar content being viewed by others
References
Albers S V, van de Vossenberg J L, Driessen A J, Konings W N. 2000. Adaptions of the archaea cell membrane to heat stress. Front Biosci, 5: 813–820
Alonso-Sáez L, Waller A S, Mende D R, Bakker K, Farnelid H, Yager P L, Lovejoy C, Tremblay J É, Potvin M, Heinrich F, Estrada M, Riemann L, Bork P, Pedrós-Alió C, Bertilsson S. 2012. Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci USA, 109: 17989–17994
Basse A, Zhu C, Versteegh G J M, Fischer G, Hinrichs K U, Mollenhauer G. 2014. Distribution of intact and core tetraether lipids in water column profiles of suspended particulate matter off Cape Blanc, NW Africa. Org Geochem, 72: 1–13
Bauersachs T, Weidenbach K, Schmitz R A, Schwark L. 2015. Distribution of glycerol ether lipids in halophilic, methanogenic and hyperthermo-philic archaea. Org Geochem, 83-84: 101–108
Bayer B, Vojvoda J, Reinthaler T, Reyes C, Pinto M, Herndl G J. 2019. Nitrosopumilus adriaticus sp. nov. and Nitrosopumilus piranensis sp. nov., two ammonia-oxidizing archaea from the Adriatic Sea and members of the class Nitrososphaeria. Int J Syst Evol Microbiol, 69: 1892–1902
Beney L, Gervais P. 2001. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl Microbiol Biotechnol, 57: 34–42
Bertoldo C, Grote R, Antranikian G. 2003. Extremophiles: Life in extreme environments. In: Encyclopedia of Environmental Microbiology. Ho-boken: John Wiley & Sons, Inc.
Boyd E S, Pearson A, Pi Y, Li W J, Zhang Y G, He L, Zhang C L, Geesey G G. 2011. Temperature and pH controls on glycerol dibiphytanyl glycerol tetraether lipid composition in the hyperthermophilic cre-narchaeon Acidilobus sulfurireducens. Extremophiles, 15: 59–65
Boyd E S, Hamilton T L, Wang J, He L, Zhang C L. 2013. The role of tetraether lipid composition in the adaptation of thermophilic archaea to acidity. Front Microbiol, 4: 62
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. 2008. Mesophilic crenarchaeota: Proposal for a third archaeal phylum, the Thaumarch-aeota. Nat Rev Microbiol, 6: 245–252
Budin I, de Rond Y, Chan L J G, Petzold C J, Keasling J D. 2018. Viscous control of cellular respiration by membrane lipid composition. Science, 362: 1186–1189
Cario A, Grossi V, Schaeffer P, Oger P M. 2015. Membrane homeoviscous adaptation in the piezo-hyperthermophilic archaeon Thermococcus barophilus. Front Microbiol, 6: 1152
Castro-Fernandez V, Zamora R, Herrera-Morande A, Vallejos G, Gonzalez-Ordenes F, Guixe V. 2017. Evolution, metabolism and molecular mechanisms underlying extreme adaptation of Euryarchaeota and its biotechnological potential. In: Sghaier H, Najjari A, Ghedira K, eds. Archaea. Rijeka: InTech
Cavicchioli R. 2006. Cold-adapted archaea. Nat Rev Microbiol, 4: 331–343
Dawson K S, Freeman K H, Macalady J L. 2012. Molecular characterization of core lipids from halophilic archaea grown under different salinity conditions. Org Geochem, 48: 1–8
de la Haba R R, Sánchez-Porro C, Marquez M C, Ventosa A. 2011. Taxonomy of halophiles. In: Horikoshi K, ed. Extremophiles Handbook. Tokyo: Springer Japan. 255–308
De Rosa M, Esposito E, Gambacorta A, Nicolaus B, Bu’Lock J D. 1980. Effects of temperature on ether lipid composition of Caldariella acid-ophila. Phytochemistry, 19: 827–831
De Rosa M, Gambacorta A, Nicolaus B, Bu’Lock J D. 1980. Complex lipids of Caldariella acidophila, a thermoacidophile archaebacterium. Phytochemistry, 19: 821–825
DeLong E F. 1992. Archaea in coastal marine environments.. Proc Natl Acad Sci USA, 89: 5685–5689
Elling F J, Könneke M, Lipp J S, Becker K W, Gagen E J, Hinrichs K U. 2014. Effects of growth phase on the membrane lipid composition of the thaumarchaeon Nitrosopumilus maritimus and their implications for archaeal lipid distributions in the marine environment. Geochim Cos-mochim Acta, 141: 579–597
Elling F J, Könneke M, Mußmann M, Greve A, Hinrichs K U. 2015. Influence of temperature, pH, and salinity on membrane lipid composition and TEX86 of marine planktonic thaumarchaeal isolates. Geochim Cosmochim Acta, 171: 238–255
Evans P N, Parks D H, Chadwick G L, Robbins S J, Orphan V J, Golding S D, Tyson G W. 2015. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science, 350: 434–438
Evans T W, Könneke M, Lipp J S, Adhikari R R, Taubner H, Elvert M, Hinrichs K U. 2018. Lipid biosynthesis of Nitrosopumilus maritimus dissected by lipid specific radioisotope probing (lipid-RIP) under contrasting ammonium supply. Geochim Cosmochim Acta, 242: 51–63
Feyhl-Buska J, Chen Y, Jia C, Wang J X, Zhang C L, Boyd E S. 2016. Influence of growth phase, pH, and temperature on the abundance and composition of tetraether lipids in the thermoacidophile Picrophilus torridus. Front Microbiol, 7: 1323
Finkel S E. 2006. Long-term survival during stationary phase: Evolution and the GASP phenotype. Nat Rev Microbiol, 4: 113–120
Franzmann P D, Stackebrandt E, Sanderson K, Volkman J K, Cameron D E, Stevenson P L, Mcmeekin T A, Burton H R. 1988. Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep Lake, Antarctica. Syst Appl Microbiol, 11: 20–27
Franzmann P D, Springer N, Ludwig W, Conway De Macario E, Rohde M. 1992. A methanogenic archaeon from Ace Lake, Antarctica: Metha-nococcoides burtonii sp. nov. Syst Appl Microbiol, 15: 573–581
Fuhrman J A, McCallum K, Davis A A. 1992. Novel major archaebacterial group from marine plankton. Nature, 356: 148–149
Gagen E J, Yoshinaga M Y, Garcia Prado F, Hinrichs K U, Thomm M. 2016. The proteome and lipidome of Thermococcus kodakarensis across the stationary phase. Archaea, 2016: 5938289
Gao J, Ward D F, Kelly R M. 2003. Hyperthermophiles. In: Bitton G, ed. Encyclopedia of Environmental Microbiology. New York: Wiley
Gibson J A E, Miller M R, Davies N W, Neill G P, Nichols D S, Volkman J K. 2005. Unsaturated diether lipids in the psychrotrophic archaeon Halorubrum lacusprofundi. Syst Appl Microbiol, 28: 19–26
Gliozzi A, Paoli G, De Rosa M, Gambacorta A. 1983. Effect of isoprenoid cyclization on the transition temperature of lipids in thermophilic ar-chaebacteria. Biochim Biophys Acta, 735: 234–242
Hallam S J, Konstantinidis K T, Putnam N, Schleper C, Watanabe Y, Sugahara J, Preston C, de la Torre J, Richardson P M, DeLong E F. 2006. Genomic analysis of the uncultivated marine crenarchaeote Cenarchaeum symbiosum. Proc Natl Acad Sci USA, 103: 18296–18301
Ho S L, Laepple T. 2016. Flat meridional temperature gradient in the early Eocene in the subsurface rather than surface ocean. Nat Geosci, 9: 606- 610
Huguet C, Kim J H, de Lange G J, Sinninghe Damsté J S, Schouten S. 2009. Effects of long term oxic degradation on the U37K, TEX86 and BIT organic proxies. Org Geochem, 40: 1188–1194
Hurley S J, Elling F J, Könneke M, Buchwald C, Wankel S D, Santoro A E, Lipp J S, Hinrichs K U, Pearson A. 2016. Influence of ammonia oxidation rate on thaumarchaeal lipid composition and the TEX86 temperature proxy. Proc Natl Acad Sci USA, 113: 7762–7767
Jain S, Caforio A, Driessen A J M. 2014. Biosynthesis of archaeal membrane ether lipids. Front Microbiol, 5: 641
Jensen S M, Neesgaard V L, Skjoldbjerg S L N, Brandl M, Ejsing C S, Treusch A H. 2015. The effects of temperature and growth phase on the lipidomes of Sulfolobus islandicus and Sulfolobus tokodaii. Life, 5: 1539–1566
Jones D L, Baxter B K. 2017. DNA repair and photoprotection: Mechanisms of overcoming environmental ultraviolet radiation exposure in halophilic archaea. Front Microbiol, 8: 1882
Kellermann M Y, Yoshinaga M Y, Valentine R C, Wörmer L, Valentine D L. 2016. Important roles for membrane lipids in haloarchaeal bioener-getics. Biochim Biophys Acta, 1858: 2940–2956
Kellermann M Y, Yoshinaga M Y, Wegener G, Krukenberg V, Hinrichs K U. 2016. Tracing the production and fate of individual archaeal intact polar lipids using stable isotope probing. Org Geochem, 95: 13–20
Kim J G, Park S J, Sinninghe Damsté J S, Schouten S, Rijpstra W I C, Jung M Y, Kim S J, Gwak J H, Hong H, Si O J, Lee S H, Madsen E L, Rhee S K. 2016. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing archaea. Proc Natl Acad Sci USA, 113: 7888–7893
Kim J H, Huguet C, Zonneveld K A F, Versteegh G J M, Roeder W, Sinninghe Damsté J S, Schouten S. 2009. An experimental field study to test the stability of lipids used for the TEX86 and U37K pa-laeothermometers. Geochim Cosmochim Acta, 73: 2888–2898
Kim J H, van der Meer J, Schouten S, Helmke P, Willmott V, Sangiorgi F, Koç N, Hopmans E C, Damsté J S S. 2010. New indices and calibrations derived from the distribution of crenarchaeal isoprenoid tetraether lipids: Implications for past sea surface temperature reconstructions. Geochim Cosmochim Acta, 74: 4639–4654
Kish A, DiRuggiero J. 2012. DNA replication and repair in halophiles. In: Vreeland R H, ed. Advances in Understanding the Biology of Halo-philic Microorganisms. Dordrecht: Springer Netherlands. 163–198
Kitzinger K, Padilla C C, Marchant H K, Hach P F, Herbold C W, Kidane A T, Könneke M, Littmann S, Mooshammer M, Niggemann J, Petrov S, Richter A, Stewart F J, Wagner M, Kuypers M M M, Bristow L A. 2019. Cyanate and urea are substrates for nitrification by Thaumarch-aeota in the marine environment. Nat Microbiol, 4: 234–243
Koga Y. 2012. Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea, 2012: 789652
Konings W N, Albers S V, Koning S, Driessen A J M. 2002. The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments. Antonie van Leeuwenhoek, 81: 61–72
Könneke M, Bernhard A E, de la Torre J R, Walker C B, Waterbury J B, Stahl D A. 2005. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature, 437: 543–546
Koyanagi T, Leriche G, Onofrei D, Holland G P, Mayer M, Yang J. 2016. Cyclohexane rings reduce membrane permeability to small ions in ar-chaea-inspired tetraether lipids. Angew Chem Int Ed, 55: 1890–1893
Lai D, Springstead J R, Monbouquette H G. 2008. Effect of growth temperature on ether lipid biochemistry in Archaeoglobus fulgidus. Ex-tremophiles, 12: 271–278
Law K P, Zhang C L. 2019. Current progress and future trends in mass spectrometry-based archaeal lipidomics. Org Geochem, 134: 45–61
Lincoln S A, Wai B, Eppley J M, Church M J, Summons R E, DeLong E F. 2014. Planktonic Euryarchaeota are a significant source of archaeal tetraether lipids in the ocean. Proc Natl Acad Sci USA, 111: 9858–9863
Liu X, Lipp J S, Hinrichs K U. 2011. Distribution of intact and core GDGTs in marine sediments. Org Geochem, 42: 368–375
Lobasso S, Pérez-Davó A, Vitale R, Sánchez M M, Corcelli A. 2015. Deciphering archaeal glycolipids of an extremely halophilic archaeon of the genus Halobellus by MALDI-TOF/MS. Chem Phys Lipids, 186: 1- 8
Lutz R A, Kennish M J. 1993. Ecology of deep-sea hydrothermal vent communities: A review. Rev Geophys, 31: 211–242
Macario A J L, Lange M, Ahring B K, De Macario E C. 1999. Stress genes and proteins in the archaea. Microbiol Mol Biol Rev, 63: 923–967
Marteinsson V T, Birrien J L, Reysenbach A L, Vernet M, Marie D, Gambacorta A, Messner P, Sleytr U B, Prieur D. 1999. Thermococcus barophilus sp. nov., a new barophilic and hyperthermophilic archaeon isolated under high hydrostatic pressure from a deep-sea hydrothermal vent. Int J Syst Bacteriol, 49: 351–359
Matsuno Y, Sugai A, Higashibata H, Fukuda W, Ueda K, Uda I, Sato I, Itoh T, Imanaka T, Fujiwara S. 2009. Effect of growth temperature and growth phase on the lipid composition of the archaeal membrane from Thermococcus kodakaraensis. Biosci Biotechnol Biochem, 73: 104–108
Meador T B, Gagen E J, Loscar M E, Goldhammer T, Yoshinaga M Y, Wendt J, Thomm M, Hinrichs K U. 2014. Thermococcus kodakarensis modulates its polar membrane lipids and elemental composition according to growth stage and phosphate availability. Front Microbiol, 5: 10
Mikucki J A, Han S K, Lanoil B D. 2011. Ecology of psychrophiles: Subglacial and permafrost environments. In: Horikoshi K, ed. Ex-tremophiles Handbook. Tokyo: Springer Japan. 755–775
Nichols D S, Miller M R, Davies N W, Goodchild A, Raftery M, Ca-vicchioli R. 2004. Cold adaptation in the Antarctic Archaeon Metha-nococcoides burtonii involves membrane lipid unsaturation. J Bacteriol, 186: 8508–8515
Oger P M, Cario A. 2013. Adaptation of the membrane in Archaea. Bio-phys Chem, 183(Suppl c): 42–56
Oren A. 2002. Adaptation of halophilic archaea to life at high salt concentrations. In: Läuchli A, Lüttge U, eds. Salinity: Environment-Plants-Molecules. Dordrecht: Springer Netherlands. 81–96
Oren A. 2008. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst, 4: 2
Oren A. 2011. Diversity of halophiles. In: Horikoshi K, ed. Extremophiles Handbook. Tokyo: Springer Japan. 309–325
Oren A. 2014. Taxonomy of halophilic Archaea: Current status and future challenges. Extremophiles, 18: 825–834
Pearson A, Ingalls A E. 2013. Assessing the use of archaeal lipids as marine environmental proxies. Annu Rev Earth Planet Sci, 41: 359–384
Pearson A, Hurley S J, Walter S R S, Kusch S, Lichtin S, Zhang Y G. 2016. Stable carbon isotope ratios of intact GDGTs indicate heterogeneous sources to marine sediments. Geochim Cosmochim Acta, 181: 18–35
Pineda De Castro L F, Dopson M, Friedman R. 2016. Biological membranes in extreme conditions: Simulations of anionic archaeal tetraether lipid membranes. PLoS ONE, 11: e0155287
Priscu J C, Christner B C. 2004. Earth’s Icy Biosphere. In: Bull A T, ed. Microbial Biodiversity and Bioprospecting. Washington D C: American Society for Microbiology Press. 130–145
Qin W, Amin S A, Martens-Habbena W, Walker C B, Urakawa H, Devol A H, Ingalls A E, Moffett J W, Armbrust E V, Stahl D A. 2014. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proc Natl Acad Sci USA, 111: 12504–12509
Qin W, Carlson L T, Armbrust E V, Devol A H, Moffett J W, Stahl D A, Ingalls A E. 2015. Confounding effects of oxygen and temperature on the TEX86 signature of marine Thaumarchaeota. Proc Natl Acad Sci USA, 112: 10979–10984
Qin W, Heal K R, Ramdasi R, Kobelt J N, Martens-Habbena W, Bertag-nolli A D, Amin S A, Walker C B, Urakawa H, Könneke M, Devol A H, Moffett J W, Armbrust E V, Jensen G J, Ingalls A E, Stahl D A. 2017. Nitrosopumilus maritimus gen. nov., sp. nov., Nitrosopumilus cobala-minigenes sp. nov., Nitrosopumilus oxyclinae sp. nov., and Ni-trosopumilus ureiphilus sp. nov., four marine ammonia-oxidizing archaea of the phylum Thaumarchaeota. Int J Syst Evol Microbiol, 67: 5067–5079
Ravelo A C, Hillaire-Marcel C. 2007. The use of oxygen and carbon isotopes of foraminifera in paleoceanography. In: Hillaire-Marcel C, De Vernal A, eds. Developments in Marine Geology. Amsterdam: Elsevier. 735–764
Reed C J, Lewis H, Trejo E, Winston V, Evilia C. 2013. Protein adaptations in archaeal extremophiles. Archaea, 2013: 1–14
Santoro A E, Richter R A, Dupont C L. 2019. Planktonic marine archaea. Annu Rev Mar Sci, 11: 131–158
Sarmiento F B, Leigh J A, Whitman W B. 2011. Genetic systems for hydrogenotrophic methanogens. In: Rosenzweig A C, Ragsdale S W, eds. Methods Enzymol. London: Academic Press. 43–73
Schouten S, Hopmans E C, Schefuß E, Sinninghe Damsté J S. 2002. Distributional variations in marine crenarchaeotal membrane lipids: A new tool for reconstructing ancient sea water temperatures? Earth Planet Sci Lett, 204: 265–274
Schouten S, Forster A, Panoto F E, Sinninghe Damsté J S. 2007. Towards calibration of the TEX86 palaeothermometer for tropical sea surface temperatures in ancient greenhouse worlds. Org Geochem, 38: 1537–1546
Schouten S, Hopmans E C, Sinninghe Damsté J S. 2013. The organic geochemistry of glycerol dialkyl glycerol tetraether lipids: A review. Org Geochem, 54: 19–61
Shimada H, Nemoto N, Shida Y, Oshima T, Yamagishi A. 2008. Effects of pH and temperature on the composition of polar lipids in Thermoplasma acidophilum HO-62. J Bacteriol, 190: 5404–5411
Siddiqui K S, Williams T J, Wilkins D, Yau S, Allen M A, Brown M V, Lauro F M, Cavicchioli R. 2013. Psychrophiles. Annu Rev Earth Planet Sci, 41: 87–115
Siliakus M F, van der Oost J, Kengen S W M. 2017. Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles, 21: 651–670
Sinninghe Damsté J S, Rijpstra W I C, Hopmans E C, den Uijl M J, Weijers J W H, Schouten S. 2018. The enigmatic structure of the crenarchaeol isomer. Org Geochem, 124: 22–28
Srivastava A, Kowalski G M, Callahan D L, Meikle P J, Creek D J. 2016. Strategies for extending metabolomics studies with stable isotope labelling and fluxomics. Metabolites, 6: 32
Stahl D A, de la Torre J R. 2012. Physiology and diversity of ammoniaoxidizing archaea. Annu Rev Microbiol, 66: 83–101
Stetter K O, Lauerer G, Thomm M, Neuner A. 1987. Isolation of extremely thermophilic sulfate reducers: Evidence for a novel branch of archae-bacteria. Science, 236: 822–824
Stetter K O. 1999. Extremophiles and their adaptation to hot environments. FEBS Lett, 452: 22–25
Tachdjian S, Shockley K R, Conners S B, Kelly R M. 2008. Functional genomics of stress response in extremophilic archaea. In: Blum P, ed. Archaea: New Models for Prokaryotic Biology. Norfolk, UK: Caister Academic Press
Taylor K W R, Huber M, Hollis C J, Hernandez-Sanchez M T, Pancost R D. 2013. Re-evaluating modern and palaeogene GDGT distributions: Implications for SST reconstructions. Glob Planet Change, 108: 158- 174
Tierney J E. 2014. Biomarker-based inferences of past climate: The TEX86 paleotemperature proxy. In: Turekian K K, ed. Treatise on Geochemistry. 2nd ed. Oxford: Elsevier. 379–393
Valentine D L. 2007. Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nat Rev Microbiol, 5: 316–323
Walker C B, de la Torre J R, Klotz M G, Urakawa H, Pinel N, Arp D J, Brochier-Armanet C, Chain P S G, Chan P P, Gollabgir A, Hemp J, Hügler M, Karr E A, Könneke M, Shin M, Lawton T J, Lowe T, Martens-Habbena W, Sayavedra-Soto L A, Lang D, Sievert S M, Ro-senzweig A C, Manning G, Stahl D A. 2010. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA, 107: 8818–8823
Wang J X, Xie W, Zhang Y G, Meador T B, Zhang C L. 2017. Evaluating production of cyclopentyl tetraethers by marine group II Euryarchaeota in the Pearl River Estuary and Coastal South China Sea: Potential impact on the TEX86 paleothermometer. Front Microbiol, 8: 2077
Williams T J, Lauro F M, Ertan H, Burg D W, Poljak A, Raftery M J, Cavicchioli R. 2011. Defining the response of a microorganism to temperatures that span its complete growth temperature range (-2°C to 28°C) using multiplex quantitative proteomics. Environ Microbiol, 13: 2186–2203
Wuchter C, Schouten S, Coolen M J L, Sinninghe Damsté J S. 2004. Temperature-dependent variation in the distribution of tetraether membrane lipids of marine Crenarchaeota: Implications for TEX86 paleothermometry. Paleoceanography, 19: PA4028
Wuchter C, Abbas B, Coolen M J L, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl G J, Middelburg J J, Schouten S, Sin-ninghe Damsté J S. 2006. Archaeal nitrification in the ocean. Proc Natl Acad Sci USA, 103: 12317–12322
Xie S, Liu X L, Schubotz F, Wakeham S G, Hinrichs K U. 2014. Distribution of glycerol ether lipids in the oxygen minimum zone of the eastern tropical North Pacific Ocean. Org Geochem, 71: 60–71
Yoshinaga M Y, Gagen E J, Wörmer L, Broda N K, Meador T B, Wendt J, Thomm M, Hinrichs K U. 2015. Methanothermobacter thermauto-trophicus modulates its membrane lipids in response to hydrogen and nutrient availability. Front Microbiol, 6: 5
Zeng Z, Liu X L, Wei J H, Summons R E, Welander P V. 2018. Calditol-linked membrane lipids are required for acid tolerance in Sulfolobus acidocaldarius. Proc Natl Acad Sci USA, 115: 12932–12937
Zhang C L, Xie W, Martin-Cuadrado A B, F. 2015. Marine group II archaea, potentially important players in the global ocean carbon cycle. Front Microbiol, 6: 1108
Zhang Y G, Pagani M, Wang Z. 2016. Ring index: A new strategy to evaluate the integrity of TEX86 paleothermometry. Paleoceanography, 31: 220–232
Zhang Y G, Liu X. 2018. Export depth of the TEX86 signal. Paleoceanogr Paleoclimatol, 33: 666–671
Acknowledgements
Special thanks to Tommy J. Phelps and Susan Pfiffner, who provided valuable comments that significantly improved the quality of the paper. This work was supported by National Key R & D Program of China (Grant Nos. 2016YFA0601101 & 2018YFA0605800), the National Natural Science Foundation of China (Grants Nos. 91851210, 41530105 & 41806085), the Key Project of Natural Science Foundation of Guangdong Province (Grants No. 2018B030311016), the Shenzhen Key Laboratory of Marine Archaea Geo-Omics, Southern University of Science and Technology (Grant No. ZDSYS201802081843490), Shenzhen International Collaboration Project (Grant No. GJHZ20180928155004783), and the Laboratory for Marine Geology, Qingdao National Laboratory for Marine Science and Technology (Grant No. MGQNLM-TD201810).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Law, K.P., Li, X. & Zhang, C. Lipidomics in archaeal membrane adaptation to environmental stresses and growth conditions: A review of culture-based physiological studies. Sci. China Earth Sci. 63, 790–807 (2020). https://doi.org/10.1007/s11430-019-9571-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11430-019-9571-2