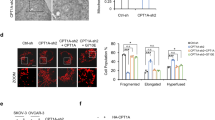
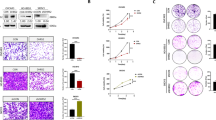
Abstract
Epithelial ovarian cancer (EOC) exhibits strong dependency on the tricarboxylic acid (TCA) cycle and oxidative phosphorylation to fuel anabolic process. Here, we show that malate dehydrogenase 2 (MDH2), a key enzyme of the TCA cycle, is palmitoylated at cysteine 138 (C138) residue, resulting in increased activity of MDH2. We next identify that ZDHHC18 acts as a palmitoyltransferase of MDH2. Glutamine deprivation enhances MDH2 palmitoylation by increasing the binding between ZDHHC18 and MDH2. MDH2 silencing represses mitochondrial respiration as well as ovarian cancer cell proliferation both in vitro and in vivo. Intriguingly, re-expression of wild-type MDH2, but not its palmitoylation-deficient C138S mutant, sustains mitochondrial respiration and restores the growth as well as clonogenic capability of ovarian cancer cells. Notably, MDH2 palmitoylation level is elevated in clinical cancer samples from patients with high-grade serous ovarian cancer. These observations suggest that MDH2 palmitoylation catalyzed by ZDHHC18 sustains mitochondrial respiration and promotes the malignancy of ovarian cancer, yielding possibilities of targeting ZDHHC18-mediated MDH2 palmitoylation in the treatment of EOC.
Similar content being viewed by others
References
Abrami, L., Kunz, B., Iacovache, I., and van der Goot, F.G. (2008). Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci USA 105, 5384–5389.
Bowtell, D.D., Böhm, S., Ahmed, A.A., Aspuria, P.J., Bast Jr, R.C., Beral, V., Berek, J.S., Birrer, M.J., Blagden, S., Bookman, M.A., et al. (2015). Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 15, 668–679.
Calsina, B., Currás-Freixes, M., Buffet, A., Pons, T., Contreras, L., Letón, R., Comino-Méndez, I., Remacha, L., Calatayud, M., Obispo, B., et al. (2018). Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genet Med 20, 1652–1662.
Cao, Y., Qiu, T., Kathayat, R.S., Azizi, S.A., Thorne, A.K., Ahn, D., Fukata, Y., Fukata, M., Rice, P.A., and Dickinson, B.C. (2019). ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5. Nat Chem Biol 15, 1232–1240.
Cascón, A., Comino-Méndez, I., Currás-Freixes, M., de Cubas, A.A., Contreras, L., Richter, S., Peitzsch, M., Mancikova, V., Inglada-Pérez, L., Pérez-Barrios, A., et al. (2015). Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J Natl Cancer Inst 107.
Chen, S., Zhu, B., Yin, C., Liu, W., Han, C., Chen, B., Liu, T., Li, X., Chen, X., Li, C., et al. (2017a). Palmitoylation-dependent activation of MC1R prevents melanomagenesis. Nature 549, 399–403.
Chen, X., Ma, H., Wang, Z., Zhang, S., Yang, H., and Fang, Z. (2017b). EZH2 palmitoylation mediated by ZDHHC5 in p53-mutant glioma drives malignant development and progression. Cancer Res 77, 4998–5010.
Chen, X., Hu, L., Yang, H., Ma, H., Ye, K., Zhao, C., Zhao, Z., Dai, H., Wang, H., and Fang, Z. (2019). DHHC protein family targets different subsets of glioma stem cells in specific niches. J Exp Clin Cancer Res 38, 25.
Corvi, M.M., Soltys, C.L.M., and Berthiaume, L.G. (2001). Regulation of mitochondrial carbamoyl-phosphate synthetase 1 activity by active site fatty acylation. J Biol Chem 276, 45704–45712.
Cuiffo, B., and Ren, R. (2010). Palmitoylation of oncogenic NRAS is essential for leukemogenesis. Blood 115, 3598–3605.
Dahl, E.S., Buj, R., Leon, K.E., Newell, J.M., Imamura, Y., Bitler, B.G., Snyder, N.W., and Aird, K.M. (2019). Targeting IDH1 as a prosenescent therapy in high-grade serous ovarian cancer. Mol Cancer Res 17, 1710–1720.
DeBerardinis, R.J., Lum, J.J., Hatzivassiliou, G., and Thompson, C.B. (2008). The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7, 11–20.
DeBerardinis, R.J., Mancuso, A., Daikhin, E., Nissim, I., Yudkoff, M., Wehrli, S., and Thompson, C.B. (2007). Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104, 19345–19350.
Dekker, F.J., Rocks, O., Vartak, N., Menninger, S., Hedberg, C., Balamurugan, R., Wetzel, S., Renner, S., Gerauer, M., Schölermann, B., et al. (2010). Small-molecule inhibition of APT1 affects Ras localization and signaling. Nat Chem Biol 6, 449–456.
Duncan, J.A., and Gilman, A.G. (1998). A cytoplasmic acyl-protein thioesterase that removes palmitate from G protein a subunits and p21RAS. J Biol Chem 273, 15830–15837.
Eisenhauer, E.A. (2017). Real-world evidence in the treatment of ovarian cancer. Ann Oncol 28, viii61–viii65.
Fantin, V.R., St-Pierre, J., and Leder, P. (2006). Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9, 425–434.
Fröhlich, M., Dejanovic, B., Kashkar, H., Schwarz, G., and Nussberger, S. (2014). S-palmitoylation represents a novel mechanism regulating the mitochondrial targeting of BAX and initiation of apoptosis. Cell Death Dis 5, e1057.
Fukata, M., Fukata, Y., Adesnik, H., Nicoll, R.A., and Bredt, D.S. (2004). Identification of PSD-95 palmitoylating enzymes. Neuron 44, 987–996.
Gatenby, R.A., and Gillies, R.J. (2004). Why do cancers have high aerobic glycolysis? Nat Rev Cancer 4, 891–899.
Gentric, G., Kieffer, Y., Mieulet, V., Goundiam, O., Bonneau, C., Nemati, F., Hurbain, I., Raposo, G., Popova, T., Stern, M.H., et al. (2019). PML-regulated mitochondrial metabolism enhances chemosensitivity in human ovarian cancers. Cell Metab 29, 156–173.e10.
Goward, C.R., and Nicholls, D.J. (1994). Malate dehydrogenase: a model for structure, evolution, and catalysis. Protein Sci 3, 1883–1888.
Guo, J., Zhang, Q., Su, Y., Lu, X., Wang, Y., Yin, M., Hu, W., Wen, W., and Lei, Q.Y. (2020). Arginine methylation of ribose-5-phosphate isomerase A senses glucose to promote human colorectal cancer cell survival. Sci China Life Sci 63, 1394–1405.
Hannoush, R.N., and Arenas-Ramirez, N. (2009). Imaging the lipidome: ω-alkynyl fatty acids for detection and cellular visualization of lipid-modified proteins. ACS Chem Biol 4, 581–587.
Hjerpe, E., Egyhazi Brage, S., Carlson, J., Frostvik Stolt, M., Schedvins, K., Johansson, H., Shoshan, M., and Avall-Lundqvist, E. (2013). Metabolic markers GAPDH, PKM2, ATP5B and BEC-index in advanced serous ovarian cancer. BMC Clin Pathol 13, 30.
Jia, D., Park, J.H., Jung, K.H., Levine, H., and Kaipparettu, B.A. (2018). Elucidating the metabolic plasticity of cancer: mitochondrial reprogramming and hybrid metabolic states. Cells 7, 21.
Jiang, H., Zhang, X., Chen, X., Aramsangtienchai, P., Tong, Z., and Lin, H. (2018). Protein lipidation: occurrence, mechanisms, biological functions, and enabling technologies. Chem Rev 118, 919–988.
Jin, J., Zhi, X., Wang, X., and Meng, D. (2021). Protein palmitoylation and its pathophysiological relevance. J Cell Physiol 236, 3220–3233.
Kathayat, R.S., Cao, Y., Elvira, P.D., Sandoz, P.A., Zaballa, M.E., Springer, M.Z., Drake, L.E., Macleod, K.F., van der Goot, F.G., and Dickinson, B. C. (2018). Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat Commun 9, 334.
Ko, P.J., and Dixon, S.J. (2018). Protein palmitoylation and cancer. EMBO Rep 19.
Kostiuk, M.A., Corvi, M.M., Keller, B.O., Plummer, G., Prescher, J.A., Hangauer, M.J., Bertozzi, C.R., Rajaiah, G., Falck, J.R., and Berthiaume, L.G. (2008). Identification of palmitoylated mitochondrial proteins using a bio-orthogonal azido-palmitate analogue. FASEB J 22, 721–732.
Lin, D.T.S., and Conibear, E. (2015). ABHD17 proteins are novel protein depalmitoylases that regulate N-Ras palmitate turnover and subcellular localization. eLife 4, e11306.
Liu, Q., Harvey, C.T., Geng, H., Xue, C., Chen, V., Beer, T.M., and Qian, D.Z. (2013). Malate dehydrogenase 2 confers docetaxel resistance via regulations of JNK signaling and oxidative metabolism. Prostate 73, 1028–1037.
Lo, Y.W., Lin, S.T., Chang, S.J., Chan, C.H., Lyu, K.W., Chang, J.F., May, E.W.S., Lin, D.Y., Chou, H.C., and Chan, H.L. (2015). Mitochondrial proteomics with siRNA knockdown to reveal ACAT1 and MDH2 in the development of doxorubicin-resistant uterine cancer. J Cell Mol Med 19, 744–759.
Long, J.Z., and Cravatt, B.F. (2011). The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem Rev 111, 6022–6063.
Lu, Y., Zheng, Y., Coyaud, É., Zhang, C., Selvabaskaran, A., Yu, Y., Xu, Z., Weng, X., Chen, J.S., Meng, Y., et al. (2019). Palmitoylation of NOD1 and NOD2 is required for bacterial sensing. Science 366, 460–467.
Matassa, D.S., Amoroso, M.R., Lu, H., Avolio, R., Arzeni, D., Procaccini, C., Faicchia, D., Maddalena, F., Simeon, V., Agliarulo, I., et al. (2016). Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ 23, 1542–1554.
Maynard, T.M., Meechan, D.W., Dudevoir, M.L., Gopalakrishna, D., Peters, A.Z., Heindel, C.C., Sugimoto, T.J., Wu, Y., Lieberman, J.A., and Lamantia, A.S. (2008). Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Mol Cell Neurosci 39, 439–451.
Morrison, E., Wegner, T., Zucchetti, A.E., Álvaro-Benito, M., Zheng, A., Kliche, S., Krause, E., Brügger, B., Hivroz, C., and Freund, C. (2020). Dynamic palmitoylation events following T-cell receptor signaling. Commun Biol 3, 368.
Nayak, A.P., Kapur, A., Barroilhet, L., and Patankar, M.S. (2018). Oxidative phosphorylation: a target for novel therapeutic strategies against ovarian cancer. Cancers 10, 337.
Ohno, Y., Kihara, A., Sano, T., and Igarashi, Y. (2006). Intracellular localization and tissue-specific distribution of human and yeast DHHC cysteine-rich domain-containing proteins. Biochim Biophys Acta 1761, 474–483.
Pan, M., Reid, M.A., Lowman, X.H., Kulkarni, R.P., Tran, T.Q., Liu, X., Yang, Y., Hernandez-Davies, J.E., Rosales, K.K., Li, H., et al. (2016). Regional glutamine deficiency in tumours promotes dedifferentiation through inhibition of histone demethylation. Nat Cell Biol 18, 1090–1101.
Pastò, A., Bellio, C., Pilotto, G., Ciminale, V., Silic-Benussi, M., Guzzo, G., Rasola, A., Frasson, C., Nardo, G., Zulato, E., et al. (2014). Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget 5, 4305–4319.
Perez, C.J., Mecklenburg, L., Jaubert, J., Martinez-Santamaria, L., Iritani, B.M., Espejo, A., Napoli, E., Song, G., Del Río, M., DiGiovanni, J., et al. (2015). Increased susceptibility to skin carcinogenesis associated with a spontaneous mouse mutation in the palmitoyl transferase Zdhhc13 gene. J Invest Dermatol 135, 3133–3143.
Prasad, P., Ghosh, S., and Roy, S.S. (2021). Glutamine deficiency promotes stemness and chemoresistance in tumor cells through DRP1-induced mitochondrial fragmentation. Cell Mol Life Sci 78, 4821–4845.
Reisch, A.S., and Elpeleg, O. (2007). Biochemical assays for mitochondrial activity: assays of TCA cycle enzymes and PDHc. In: Methods in Cell Biology. New York: Academic Press. 199–222.
Schroeder, H., Leventis, R., Rex, S., Schelhaas, M., Nägele, E., Waldmann, H., and Silvius, J.R. (1997). S-acylation and plasma membrane targeting of the farnesylated carboxyl-terminal peptide of N-ras in mammalian fibroblasts. Biochemistry 36, 13102–13109.
Shen, L.F., Chen, Y.J., Liu, K.M., Haddad, A.N.S., Song, I.W., Roan, H.Y., Chen, L.Y., Yen, J.J.Y., Chen, Y.J., Wu, J.Y., et al. (2017). Role of S-palmitoylation by ZDHHC13 in mitochondrial function and metabolism in liver. Sci Rep 7, 2182.
Small, W.C., and McAlister-Henn, L. (1997). Metabolic effects of altering redundant targeting signals for yeast mitochondrial malate dehydrogenase. Arch Biochem Biophys 344, 53–60.
Sun, H. W., Wu, W.C., Chen, H. T., Xu, Y. T., Yang, Y. Y., Chen, J., Yu, X. J., Wang, Z., Shuang, Z.Y., and Zheng, L. (2020). Glutamine deprivation promotes the generation and mobilization of MDSCs by enhancing expression of G-CSF and GM-CSF. Front Immunol 11, 616367.
Vögtle, F.N., Burkhart, J.M., Gonczarowska-Jorge, H., Kücükköse, C., Taskin, A.A., Kopczynski, D., Ahrends, R., Mossmann, D., Sickmann, A., Zahedi, R.P., et al. (2017). Landscape of submitochondrial protein distribution. Nat Commun 8, 290.
Wagner, G.R., Bhatt, D.P., O’Connell, T.M., Thompson, J.W., Dubois, L.G., Backos, D.S., Yang, H., Mitchell, G.A., Ilkayeva, O.R., Stevens, R.D., et al. (2017). A class of reactive acyl-CoA species reveals the non-enzymatic origins of protein acylation. Cell Metab 25, 823–837.e8.
Yang, C., Ko, B., Hensley, C.T., Jiang, L., Wasti, A.T., Kim, J., Sudderth, J., Calvaruso, M.A., Lumata, L., Mitsche, M., et al. (2014a). Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol Cell 56, 414–424.
Yang, L., Moss, T., Mangala, L.S., Marini, J., Zhao, H., Wahlig, S., Armaiz-Pena, G., Jiang, D., Achreja, A., Win, J., et al. (2014b). Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol 10, 728.
Yao, H., Lan, J., Li, C., Shi, H., Brosseau, J.P., Wang, H., Lu, H., Fang, C., Zhang, Y., Liang, L., et al. (2019). Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours. Nat Biomed Eng 3, 306–317.
Yeste-Velasco, M., Mao, X., Grose, R., Kudahetti, S.C., Lin, D., Marzec, J., Vasiljevic, N., Chaplin, T., Xue, L., Xu, M., et al. (2014). Identification of ZDHHC14 as a novel human tumour suppressor gene. J Pathol 232, 566–577.
Yu, L., Lu, M., Jia, D., Ma, J., Ben-Jacob, E., Levine, H., Kaipparettu, B.A., and Onuchic, J.N. (2017). Modeling the genetic regulation of cancer metabolism: interplay between glycolysis and oxidative phosphorylation. Cancer Res 77, 1564–1574.
Yuan, M., Chen, X., Sun, Y., Jiang, L., Xia, Z., Ye, K., Jiang, H., Yang, B., Ying, M., Cao, J., et al. (2020). ZDHHC12-mediated claudin-3 S-palmitoylation determines ovarian cancer progression. Acta Pharm Sin B 10, 1426–1439.
Zacksenhaus, E., Shrestha, M., Liu, J.C., Vorobieva, I., Chung, P.E.D., Ju, Y.J., Nir, U., and Jiang, Z. (2017). Mitochondrial OXPHOS induced by RB1 deficiency in breast cancer: implications for anabolic metabolism, stemness, and metastasis. Trends Cancer 3, 768–779.
Zhang, X., Zhang, L., Ji, G., Lei, Q., Fang, C., and Lu, H. (2018). Site-specific quantification of protein palmitoylation by cysteine-stable isotope metabolic labeling. Anal Chem 90, 10543–10550.
Zhao, S., Xu, W., Jiang, W., Yu, W., Lin, Y., Zhang, T., Yao, J., Zhou, L., Zeng, Y., Li, H., et al. (2010). Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2020YFA0803402 and 2019YFA0801703), the National Natural Science Foundation of China (81872240, 81802745, 81790250/81790253 and 91959202), and Innovation Program of Shanghai Municipal Education Commission (N173606). We thank members of the Lei Laboratory for discussion throughout this study. We also appreciate the Biomedical Core Facility of Fudan University for technical support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Compliance and ethics The author(s) declare that they have no conflict of interest. The procedures related to human and animal subjects of our study were approved by Ethic Committee of Fudan University Shanghai Cancer Center and Ethics Committee of the Department of Laboratory Animals, Fudan University, China.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Pei, X., Li, KY., Shen, Y. et al. Palmitoylation of MDH2 by ZDHHC18 activates mitochondrial respiration and accelerates ovarian cancer growth. Sci. China Life Sci. 65, 2017–2030 (2022). https://doi.org/10.1007/s11427-021-2048-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-021-2048-2