Abstract
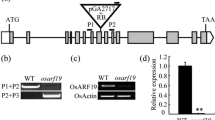
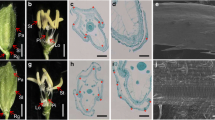
The palea and lemma are floral organ structures unique to grasses; these structures form the hull and directly affect grain size and quality. However, the molecular mechanisms controlling the development of the hull are not well understood. In this study, we characterized the rice (Oryza sativa) abnormal flower and grain1 (afg1) mutant, a new allele of OsMADS6. Similar to previously characterized osmads6 alleles, in the afg1 floret, the palea lost its marginal region and acquired the lemma identity. However, in contrast to other osmads6 alleles, the afg1 mutant showed altered grain size and grain quality, with decreased total starch and amylose contents, and increased protein and soluble sugar contents. The analysis of transcriptional activity suggested that AFG1 is a transcriptional activator and may affect grain size by regulating the expression levels of several genes related to cell expansion and proliferation in the afg1 mutant. These results revealed that AFG1 plays an important role in determining palea identity and affecting grain yield and quality in rice.
Similar content being viewed by others
References
Angenent, G.C., Franken, J., Busscher, M., van Dijken, A., van Went, J.L., Dons, H.J., and van Tunen, A.J. (1995). A novel class of MADS box genes is involved in ovule development in petunia.. Plant Cell 7, 1569–1582.
Arora, R., Agarwal, P., Ray, S., Singh, A.K., Singh, V.P., Tyagi, A.K., and Kapoor, S. (2007). MADS-box gene family in rice: genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genomics 8, 242.
Becker, A., and Theissen, G. (2003). The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol Phylogenet Evol 29, 464–489.
Cai, Y., Li, S., Jiao, G., Sheng, Z., Wu, Y., Shao, G., Xie, L., Peng, C., Xu, J., Tang, S., et al. (2018). OsPK2 encodes a plastidic pyruvate kinase involved in rice endosperm starch synthesis, compound granule formation and grain filling. Plant Biotechnol J 16, 1878–1891.
Coen, E.S., and Meyerowitz, E.M. (1991). The war of the whorls: genetic interactions controlling flower development. Nature 353, 31–37.
Cheng, L., Shafiq, S., Xu, W., and Sun, Q. (2018). EARLY FLOWERING IN SHORT DAYS (EFS) regulates the seed size in Arabidopsis. Sci China Life Sci 61, 214–224.
Cui, R., Han, J., Zhao, S., Su, K., Wu, F., Du, X., Xu, Q., Chong, K., Theissen, G., and Meng, Z. (2010). Functional conservation and diversification of class E floral homeotic genes in rice (Oryza sativa). Plant J 61, 767–781.
Ditta, G., Pinyopich, A., Robles, P., Pelaz, S., and Yanofsky, M.F. (2004). The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14, 1935–1940.
Dreni, L., Jacchia, S., Fornara, F., Fornari, M., Ouwerkerk, P.B.F., An, G., Colombo, L., and Kater, M.M. (2007). The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J 52, 690–699.
Duan, P., Rao, Y., Zeng, D., Yang, Y., Xu, R., Zhang, B., Dong, G., Qian, Q., and Li, Y. (2014). SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J 77, 547–557.
Fang, N., Xu, R., Huang, L., Zhang, B., Duan, P., Li, N., Luo, Y., and Li, Y. (2016). SMALL GRAIN 11 controls grain size, grain number and grain yield in rice. Rice 9, 64.
Ferrario, S., Immink, R.G.H., and Angenent, G.C. (2004). Conservation and diversity in flower land. Curr Opin Plant Biol 7, 84–91.
Gao, X., Liang, W., Yin, C., Ji, S., Wang, H., Su, X., Guo, C., Kong, H., Xue, H., and Zhang, D. (2010). The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol 153, 728–740.
Hu, J., Wang, Y., Fang, Y., Zeng, L., Xu, J., Yu, H., Shi, Z., Pan, J., Zhang, D., Kang, S., et al. (2015). A rare allele of GS2 enhances grain size and grain yield in rice. Mol Plant 8, 1455–1465.
Jeon, J.S., Jang, S., Lee, S., Nam, J., Kim, C., Lee, S.H., Chung, Y.Y., Kim, S.R., Lee, Y.H., Cho, Y.G., et al. (2000). leafy hull sterile1 is a homeotic mutation in a rice MADS box gene affecting rice flower development. Plant Cell 12, 871–884.
Jin, Y., Luo, Q., Tong, H., Wang, A., Cheng, Z., Tang, J., Li, D., Zhao, X., Li, X., Wan, J., et al. (2011). An AT-hook gene is required for palea formation and floral organ number control in rice. Dev Biol 359, 277–288.
Kang, H.G., Park, S., Matsuoka, M., and An, G. (2005). White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB). Plant J 42, 901–911.
Li, H., Liang, W., Jia, R., Yin, C., Zong, J., Kong, H., and Zhang, D. (2009). The AGL6-like gene OsMADS6 regulates floral organ and meristem identities in rice. Cell Res 20, 299–313.
Lin, X., Wu, F., Du, X., Shi, X., Liu, Y., Liu, S., Hu, Y., Theißen, G., and Meng, Z. (2013). The pleiotropic SEPALLATA-like gene OsMADS34 reveals that the ‘empty glumes’ of rice (Oryza sativa) spikelets are in fact rudimentary lemmas. New Phytol 202, 689–702.
Liu, L., Ma, X., Liu, S., Zhu, C., Jiang, L., Wang, Y., Shen, Y., Ren, Y., Dong, H., Chen, L., et al. (2009). Identification and characterization of a novel Waxy allele from a Yunnan rice landrace. Plant Mol Biol 71, 609–626.
Luo, Q., Zhou, K., Zhao, X., Zeng, Q., Xia, H., Zhai, W., Xu, J., Wu, X., Yang, H., and Zhu, L. (2005). Identification and fine mapping of a mutant gene for palealess spikelet in rice. Planta 221, 222–230.
Malcomber, S.T., and Kellogg, E.A. (2004). Heterogeneous expression patterns and separate roles of the SEPALLATA gene LEAFY HULL STERILE1 in grasses. Plant Cell 16, 1692–1706.
Meng, J.G., Zhang, M.X., Yang, W.C., and Li, H.J. (2019). TICKET attracts pollen tubes and mediates reproductive isolation between relative species in Brassicaceae. Sci China Life Sci 62, 1413–1419.
Nagasawa, N., Miyoshi, M., Sano, Y., Satoh, H., Hirano, H., Sakai, H., and Nagato, Y. (2003). SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130, 705–718.
Nardmann, J., Ji, J., Werr, W., and Scanlon, M.J. (2004). The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development 131, 2827–2839.
Nishi, A., Nakamura, Y., Tanaka, N., and Satoh, H. (2001). Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol 127, 459–472.
Norman, C., Runswick, M., Pollock, R., and Treisman, R. (1988). Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-Fos serum response element. Cell 55, 989–1003.
Ohmori, S., Kimizu, M., Sugita, M., Miyao, A., Hirochika, H., Uchida, E., Nagato, Y., and Yoshida, H. (2009). MOSAIC FLORAL ORGANS1, an AGL6-like MADS box gene, regulates floral organ identity and meristem fate in rice. Plant Cell 21, 3008–3025.
Pelaz, S., Tapia-López, R., Alvarez-Buylla, E.R., and Yanofsky, M.F. (2001a). Conversion of leaves into petals in Arabidopsis. Curr Biol 11, 182–184.
Pelaz, S., Gustafson-Brown, C., Kohalmi, S.E., Crosby, W.L., and Yanofsky, M.F. (2001b). APETALA1 and SEPALLATA3 interact to promote flower development. Plant J 26, 385–394.
Pelucchi, N., Fornara, F., Favalli, C., Masiero, S., Lago, C., Pè, E.M., Colombo, L., and Kater, M.M. (2002). Comparative analysis of rice MADS-box genes expressed during flower development. Sexual Plant Reprod 15, 113–122.
Prasad, K., Parameswaran, S., and Vijayraghavan, U. (2005). OsMADS1, a rice MADS-box factor, controls differentiation of specific cell types in the lemma and palea and is an early-acting regulator of inner floral organs. Plant J 43, 915–928.
Qiu, Z., Zhu, L., He, L., Chen, D., Zeng, D., Chen, G., Hu, J., Zhang, G., Ren, D., Dong, G., et al. (2019). DNA damage and reactive oxygen species cause cell death in the rice local lesions 1 mutant under high light and high temperature. New Phytol 222, 349–365.
Ren, D., Li, Y., Zhao, F., Sang, X., Shi, J., Wang, N., Guo, S., Ling, Y., Zhang, C., Yang, Z., et al. (2013). MULTI-FLORET SPIKELET1, which encodes an AP2/ERF protein, determines spikelet meristem fate and sterile lemma identity in rice. Plant Physiol 162, 872–884.
Ren, D., Yu, H., Rao, Y., Xu, Q., Zhou, T., Hu, J., Zhang, Y., Zhang, G., Zhu, L., Gao, Z., et al. (2018). ‘Two-floret spikelet’ as a novel resource has the potential to increase rice yield. Plant Biotechnol J 16, 351–353.
Ren, D., Xu, Q., Qiu, Z., Cui, Y., Zhou, T., Zeng, D., Guo, L., and Qian, Q. (2019a). FON 4 prevents the multi-floret spikelet in rice. Plant Biotechnol J 17, 1007–1009.
Ren, D., Cui, Y., Hu, H., Xu, Q., Rao, Y., Yu, X., Zhang, Y., Wang, Y., Peng, Y., Zeng, D., et al. (2019b). AH 2 encodes a MYB domain protein that determines hull fate and affects grain yield and quality in rice. Plant J 100, 813–824.
Sang, X., Li, Y., Luo, Z., Ren, D., Fang, L., Wang, N., Zhao, F., Ling, Y., Yang, Z., Liu, Y., et al. (2012). CHIMERIC FLORAL ORGANS1, encoding a monocot-specific MADS box protein, regulates floral organ identity in rice. Plant Physiol 160, 788–807.
Scanlon, M.J., Schneeberger, R.G., and Freeling, M. (1996). The maize mutant narrow sheath fails to establish leaf margin identity in a meristematic domain. Development 122, 1683–1691.
Schilling, S., Pan, S., Kennedy, A., and Melzer, R. (2018). MADS-box genes and crop domestication: the jack of all traits. J Exp Bot 69, 1447–1469.
Smaczniak, C., Immink, R.G.H., Angenent, G.C., and Kaufmann, K. (2012). Developmental and evolutionary diversity of plant MADS-domain factors: insights from recent studies. Development 139, 3081–3098.
Theissen, G., Becker, A., Di Rosa, A., Kanno, A., Kim, J.T., Münster, T., Winter, K.U., and Saedler, H. (2000). A short history of MADS-box genes in plants. Plant Mol Biol 42, 115–149.
Wang, K., Tang, D., Hong, L., Xu, W., Huang, J., Li, M., Gu, M., Xue, Y., and Cheng, Z. (2010). DEP and AFO regulate reproductive habit in rice. PLoS Genet 6, e1000818.
Yamaguchi, T., Lee, D.Y., Miyao, A., Hirochika, H., An, G., and Hirano, H. Y. (2006). Functional diversification of the two C-class MADS box genes OSMADS3 and OSMADS58 in Oryza sativa. Plant Cell 18, 15–28.
Yan, D., Zhang, X., Zhang, L., Ye, S., Zeng, L., Liu, J., Li, Q., and He, Z. (2015). CURVED CHIMERIC PALEA 1 encoding an EMF1-like protein maintains epigenetic repression of OsMADS58 in rice palea development. Plant J 82, 12–24.
Yao, S.G., Ohmori, S., Kimizu, M., and Yoshida, H. (2008). Unequal genetic redundancy of rice PISTILLATA orthologs, OsMADS2 and OsMADS4, in lodicule and stamen development. Plant Cell Physiol 49, 853–857.
Yin, D., Liu, X., Shi, Z., Li, D., and Zhu, L. (2018). An AT-hook protein DEPRESSED PALEA1 physically interacts with the TCP family transcription factor RETARDED PALEA1 in rice. Biochem Biophys Res Commun 495, 487–492.
Yu, Y., Song, J., Tian, X., Zhang, H., Li, L., and Zhu, H. (2018). Arabidopsis PRK6 interacts specifically with AtRopGEF8/12 and induces depolarized growth of pollen tubes when overexpressed. Sci China Life Sci 61, 100–112.
Yuan, Z., Gao, S., Xue, D.W., Luo, D., Li, L.T., Ding, S.Y., Yao, X., Wilson, Z.A., Qian, Q., and Zhang, D.B. (2009). RETARDED PALEA1 controls palea development and floral zygomorphy in rice. Plant Physiol 149, 235–244.
Zahn, L.M., Kong, H., Leebens-Mack, J.H., Kim, S., Soltis, P.S., Landherr, L.L., Soltis, D.E., Depamphilis, C.W., and Ma, H. (2005). The evolution of the SEPALLATA subfamily of MADS-box genes. Genetics 169, 2209–2223.
Zanis, M.J. (2007). Grass spikelet genetics and duplicate gene comparisons. Int J Plant Sci 168, 93–110.
Zhang, Y.J., Zhang, Y., Zhang, L.L., Huang, H.Y., Yang, B.J., Luan, S., Xue, H.W., and Lin, W.H. (2018). OsGATA7 modulates brassinosteroids-mediated growth regulation and influences architecture and grain shape. Plant Biotechnol J 16, 1261–1264.
Zheng, M., Wang, Y., Wang, Y., Wang, C., Ren, Y., Lv, J., Peng, C., Wu, T., Liu, K., Zhao, S., et al. (2015). DEFORMED FLORAL ORGAN1 (DFO1) regulates floral organ identity by epigenetically repressing the expression of OsMADS58 in rice (Oryza sativa). New Phytol 206, 1476–1490.
Zhou, Y., Miao, J., Gu, H., Peng, X., Leburu, M., Yuan, F., Gu, H., Gao, Y., Tao, Y., Zhu, J., et al. (2015). Natural variations in SLG7 regulate grain shape in rice. Genetics 201, 1591–1599.
Acknowledgements
This work was supported by the Zhejiang Natural Science Foundation (LY18C130007), the National Natural Science Foundation of China (91735304), the Central Public-interest Scientific Institution Basal Research Fund of China National Rice Research Institute (2017RG001-4), and the National Science and Technology Major Project (2016ZX08009003-003-008).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no conflict of interest.
Supporting Information
Rights and permissions
About this article
Cite this article
Yu, X., Xia, S., Xu, Q. et al. ABNORMAL FLOWER AND GRAIN 1 encodes OsMADS6 and determines palea identity and affects rice grain yield and quality. Sci. China Life Sci. 63, 228–238 (2020). https://doi.org/10.1007/s11427-019-1593-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11427-019-1593-0