Abstract
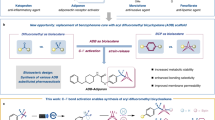
Pentafluorosulfanylated (SF5-) aromatics have shown great potential in drugs, and the bioisosteric replacement of aromatic ring with bicyclo[1.1.1]pentane (BCP) unit has attracted considerable attention recently. Consequently, pentafluorosulfanylated bicyclo[1.1.1]pentanes (SF5-BCPs) should have application in the realm of drug discovery. In this study, a one-pot iodopentafluorosulfanylation of [1.1.1]propellane with SF5Cl and CH2I2 for the practical synthesis of iodopentafluorosulfanylated bicyclo[1.1.1]pentane (SF5-BCP-I) was developed. SF5-BCP-I was the first example of SF5-BCPs that could be transformed. The first general method to access SF5-substituted bicyclo[1.1.1]pentane derivatives was demonstrated through photoredox-catalyzed radical addition of SF5-BCP-I to alkenes and alkynes.

Similar content being viewed by others
References
Muller K, Faeh C, Diederich F. Science, 2007, 317: 1881–1886
Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev, 2008, 37: 320–330
Hagmann WK. J Med Chem, 2008, 51: 4359–4369
Meanwell NA. J Med Chem, 2018, 61: 5822–5880
Savoie PR, Welch JT. Chem Rev, 2015, 115: 1130–1190
Sowaileh MF, Hazlitt RA, Colby DA. ChemMedChem, 2017, 12: 1481–1490
Kordnezhadian R, Li B, Zogu A, Demaerel J, De Borggraeve WM, Ismalaj E. Chem Eur J, 2022, 28: e202201491
Sani M, Zanda M. Synthesis, 2022, 54: 4184–4209
Macdonald JD, Chacón Simon S, Han C, Wang F, Shaw JG, Howes JE, Sai J, Yuh JP, Camper D, Alicie BM, Alvarado J, Nikhar S, Payne W, Aho ER, Bauer JA, Zhao B, Phan J, Thomas LR, Rossanese OW, Tansey WP, Waterson AG, Stauffer SR, Fesik SW. J Med Chem, 2019, 62: 11232–11259
Salamoun JM, Garcia CJ, Hargett SR, Murray JH, Chen SY, Beretta M, Alexopoulos SJ, Shah DP, Olzomer EM, Tucker SP, Hoehn KL, Santos WL. J Med Chem, 2020, 63: 6203–6224
Codony S, Pujol E, Pizarro J, Feixas F, Valverde E, Loza MI, Brea JM, Saez E, Oyarzabal J, Pineda-Lucena A, Pérez B, Pérez C, Rodríguez-Franco MI, Leiva R, Osuna S, Morisseau C, Hammock BD, Vázquez-Carrera M, Vázquez S. J Med Chem, 2020, 63: 9237–9257
Tang H, Jensen K, Houang E, McRobb FM, Bhat S, Svensson M, Bochevarov A, Day T, Dahlgren MK, Bell JA, Frye L, Skene RJ, Lewis JH, Osborne JD, Tierney JP, Gordon JA, Palomero MA, Gallati C, Chapman RSL, Jones DR, Hirst KL, Sephton M, Chauhan A, Sharpe A, Tardia P, Dechaux EA, Taylor A, Waddell RD, Valentine A, Janssens HB, Aziz O, Bloomfield DE, Ladha S, Fraser IJ, Ellard JM. J Med Chem, 2022, 65: 6775–6802
Faber EB, Wang N, John K, Sun L, Wong HL, Burban D, Francis R, Tian D, Hong KH, Yang A, Wang L, Elsaid M, Khalid H, Levinson NM, Schönbrunn E, Hawkinson JE, Georg GI. J Med Chem, 2023, 66: 1928–1940
Lovering F, Bikker J, Humblet C. J Med Chem, 2009, 52: 6752–6756
For recent reviews, see: (a) Kanazawa J, Uchiyama M. Synlett, 2019, 30: 1–11
Ma X, Nhat Pham L. Asian J Org Chem, 2020, 9: 8–22
He FS, Xie S, Yao Y, Wu J. Chin Chem Lett, 2020, 31: 3065–3072
Anderson JM, Measom ND, Murphy JA, Poole DL. Angew Chem Int Ed, 2021, 60: 24754–24769
Pramanik MMD, Qian H, Xiao WJ, Chen JR. Org Chem Front, 2020, 7: 2531–2537
Nied D, Breher F. Chem Soc Rev, 2011, 40: 3455–3466
For selected examples, see: (a) Gianatassio R, Lopchuk JM, Wang J, Pan CM, Malins LR, Prieto L, Brandt TA, Collins MR, Gallego GM, Sach NW, Spangler JE, Zhu H, Zhu J, Baran PS. Science, 2016, 351: 241–246
Kanazawa J, Maeda K, Uchiyama M. J Am Chem Soc, 2017, 139: 17791–17794
Caputo DFJ, Arroniz C, Dürr AB, Mousseau JJ, Stepan AF, Mansfield SJ, Anderson EA. Chem Sci, 2018, 9: 5295–5300
Shelp RA, Walsh PJ. Angew Chem Int Ed, 2018, 57: 15857–15861
Nugent J, Arroniz C, Shire BR, Sterling AJ, Pickford HD, Wong MLJ, Mansfield SJ, Caputo DFJ, Owen B, Mousseau JJ, Duarte F, Anderson EA. ACS Catal, 2019, 9: 9568–9574
Hughes JME, Scarlata DA, Chen ACY, Burch JD, Gleason JL. Org Lett, 2019, 21: 6800–6804
Trongsiriwat N, Pu Y, Nieves-Quinones Y, Shelp RA, Kozlowski MC, Walsh PJ. Angew Chem Int Ed, 2019, 58: 13416–13420
Kondo M, Kanazawa J, Ichikawa T, Shimokawa T, Nagashima Y, Miyamoto K, Uchiyama M. Angew Chem Int Ed, 2020, 59: 1970–1974
Zhang X, Smith RT, Le C, McCarver SJ, Shireman BT, Carruthers NI, MacMillan DWC. Nature, 2020, 580: 220–226
Kim JH, Ruffoni A, Al-Faiyz YSS, Sheikh NS, Leonori D. Angew Chem Int Ed, 2020, 59: 8225–8231
Shin S, Lee S, Choi W, Kim N, Hong S. Angew Chem Int Ed, 2021, 60: 7873–7879
Wong MLJ, Sterling AJ, Mousseau JJ, Duarte F, Anderson EA. Nat Commun, 2021, 12: 1644–1652
Wu Z, Xu Y, Liu J, Wu X, Zhu C. Sci China Chem, 2020, 63: 1025–1029
Huang W, Keess S, Molander GA. J Am Chem Soc, 2022, 144: 12961–12969
Yu IF, Manske JL, Diéguez-Vázquez A, Misale A, Pashenko AE, Mykhailiuk PK, Ryabukhin SV, Volochnyuk DM, Hartwig JF. Nat Chem, 2023, 15: 685–693
Huang W, Keess S, Molander GA. Angew Chem Int Ed, 2023, 62: e202302223
Alvarez EM, Bai Z, Pandit S, Frank N, Torkowski L, Ritter T. Nat Synth, 2023, 2: 548–556
For recent reviews, see: (a) Magre M, Ni S, Cornella J. Angew Chem Int Ed, 2022, 61: e202200904
Haufe G. Tetrahedron, 2022, 109: 132656–132692
Kraemer Y, Bergman EN, Togni A, Pitts CR. Angew Chem Int Ed, 2022, 61: e202205088
Popek L, Nguyen TM, Blanchard N, Cahard D, Bizet V. Tetrahedron, 2022, 117–118: 132814
Case JR, Ray NH, Roberts HL. J Chem Soc, 1961, 2066
Case JR, Ray NH, Roberts HL. J Chem Soc, 1961, 2070
Aït-Mohand S, Dolbier WR. Org Lett, 2002, 4: 3013–3015
Shou J, Xu X, Qing F. Angew Chem Int Ed, 2021, 60: 15271–15275
Birepinte M, Champagne PA, Paquin J. Angew Chem Intl Edit, 2022, 61: e202112575
Shou J, Qing F. Angew Chem Int Ed, 2022, 61: e202208860
Shou JY, Xu XH, Qing FL. J Fluorine Chem, 2022, 261–262: 110018
Gilbert A, Birepinte M, Paquin JF. J Fluorine Chem, 2021, 243: 109734–109743
Gilbert A, Paquin JF. J Fluorine Chem, 2019, 221: 70–74
Kraemer Y, Ghiazza C, Ragan AN, Ni S, Lutz S, Neumann EK, Fettinger JC, Nöthling N, Goddard R, Cornella J, Pitts CR. Angew Chem Int Ed, 2022, 61: e202211892
Kaszynski P, McMurdie ND, Michl J. J Org Chem, 1991, 56: 307–316
Nugent J, Shire BR, Caputo DFJ, Pickford HD, Nightingale F, Houlsby ITT, Mousseau JJ, Anderson EA. Angew Chem Int Ed, 2020, 59: 11866–11870
Pickford HD, Nugent J, Owen B, Mousseau JJ, Smith RC, Anderson EA. J Am Chem Soc, 2021, 143: 9729–9736
Yen-Pon E, Li L, Levitre G, Majhi J, McClain EJ, Voight EA, Crane EA, Molander GA. J Am Chem Soc, 2022, 144: 12184–12191
Hutchinson J. J Fluorine Chem, 1974, 3: 429–432
Deposition Number 2255639 (for 1a) contains the supplementary crystallographic data for this paper These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service
Pickford HD, Ripenko V, McNamee RE, Holovchuk S, Thompson AL, Smith RC, Mykhailiuk PK, Anderson EA. Angew Chem Int Ed, 2023, 62: e202213508
Livesley S, Trueman B, Robertson CM, Goundry WRF, Morris JA, Aïssa C. Org Lett, 2022, 24: 7015–7020
Goh YL, Tam EKW, Bernardo PH, Cheong CB, Johannes CW, William AD, Adsool VA. Org Lett, 2014, 16: 1884–1887
Zhao X, Shou JY, Newton JJ, Qing FL. Org Lett, 2022, 24: 8412–8416
Kopping B, Chatgilialoglu C, Zehnder M, Giese B. J Org Chem, 1992, 57: 3994–4000
Semmler K, Szeimies G, Belzner J. J Am Chem Soc, 1985, 107: 6410–6411
Wiberg KB, Waddell ST. J Am Chem Soc, 1990, 112: 2194–2216
Bär RM, Kirschner S, Nieger M, Bräse S. Chem Eur J, 2018, 24: 1373–1382
Bär RM, Heinrich G, Nieger M, Fuhr O, Bräse S. Beilstein J Org Chem, 2019, 15: 1172–1180
Sivaguru P, Bi X. Sci China Chem, 2021, 64: 1614–1629
Kucher H, Wenzel JO, Rombach D, ChemRxiv, 2022, doi:https://doi.org/10.26434/chemrxiv-2022-01jhn
Popek L, Cabrera-Trujillo JJ, Debrauwer V, Blanchard N, Miqueu K, Bizet V. Angew Chem Int Ed, 2023, 62: e202300685
Bao ZP, Zhang Y, Wang LC, Wu XF. Sci China Chem, 2023, 66: 139–146
Smith BR, Eastman CM, Njardarson JT. J Med Chem, 2014, 57: 9764–9773
Deposition Number 2255640 (for 6h) contains the supplementary crystallographic data for this paper These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service
For selected examples of the reaction of disulfides in the presence of copper, see: (a) Green KA, Hoover JM. ACS Catal, 2019, 10: 1769–1782
Xu W, Hei YY, Song JL, Zhan XC, Zhang XG, Deng CL. Synthesis, 2019, 51: 545–551
Wang Y, Deng J, Chen J, Cao F, Hou Y, Yang Y, Deng X, Yang J, Wu L, Shao X, Shi T, Wang Z. ACS Catal, 2020, 10: 2707–2712
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21991121) and the National Key Research and Development Program of China (2021YFF0701700).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Additional information
Supporting information The supporting information is available online at https://chem.scichina.com and https://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
Rights and permissions
About this article
Cite this article
Zhao, X., Shou, JY. & Qing, FL. Iodopentafluorosulfanylation of [1.1.1]propellane and further functionalizations. Sci. China Chem. 66, 2871–2877 (2023). https://doi.org/10.1007/s11426-023-1715-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1715-2