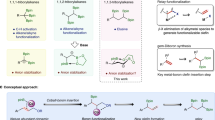
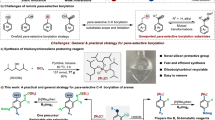
Abstract
A mild, chemoselective, redox-neutral ipso/ortho alkenylcyanation of arylboronic acids with homopropargylic malononitriles via 1,4-rhodium migration and fragmentation is reported. A variety of 2-vinyl arylnitriles are obtained in good yields (51 examples, ava. 69% yields) through this strategy, which is characterized by its broad substrate scope, great functional group tolerance, and mild conditions. Mechanism studies indicate that the fragmentation is temperature dependent. The primary asymmetric exploration for the non-fragmentation product already shows promising results. The separation of the two cyano groups of homopropargylic malononitriles results in the formation of aromatic nitrile and aliphatic nitrile in one molecule, which enables the further transformations of the products.

Similar content being viewed by others
References
Hedouin G, Hazra S, Gallou F, Handa S. ACS Catal, 2022, 12: 4918–4937
Corpas J, Mauleón P, Arrayás RG, Carretero JC. ACS Catal, 2021, 11: 7513–7551
West MJ, Fyfe JWB, Vantourout JC, Watson AJB. Chem Rev, 2019, 119: 12491–12523
Malapit CA, Bour JR, Brigham CE, Sanford MS. Nature, 2018, 563: 100–104
Miyaura N, Suzuki A. Chem Rev, 1995, 95: 2457–2483
Wang H, Jing C, Noble A, Aggarwal VK. Angew Chem Int Ed, 2020, 59: 16859–16872
Roscales S, Csáky AG. Chem Soc Rev, 2020, 49: 5159–5177
Wu P, Givskov M, Nielsen TE. Chem Rev, 2019, 119: 11245–11290
Zhu C, Falck JR. Adv Synth Catal, 2014, 356: 2395–2410
Oguma K, Miura M, Satoh T, Nomura M. J Am Chem Soc, 2000, 122: 10464–10465
Hayashi T, Inoue K, Taniguchi N, Ogasawara M. J Am Chem Soc, 2001, 123: 9918–9919
Kong W-, Finger LH, Oliveira JCA, Ackermann L. Angew Chem Int Ed, 2019, 58: 6342–6346
Xu S, Chen K, Chen H, Yao J, Zhu X. Chem Eur J, 2014, 20: 16442–16447
Karthikeyan J, Haridharan R, Cheng CH. Angew Chem Int Ed, 2012, 51: 12343–12347
Fukutani T, Hirano K, Satoh T, Miura M. J Org Chem, 2011, 76: 2867–2874
Fukutani T, Hirano K, Satoh T, Miura M. Org Lett, 2010, 11: 5198–5201
Selmani A, Darses S. Org Lett, 2020, 22: 2681–2686
Groves A, Sun J, Parke HRI, Callingham M, Argent SP, Taylor LJ, Lam HW. Chem Sci, 2020, 11: 2759–2764
Selmani A, Darses S. Org Lett, 2019, 21: 8122–8126
O’Brien L, Karad SN, Lewis W, Lam HW. Chem Commun, 2019, 55: 11366–11369
Wang JX, Tan YX, Liao WW, Tian P, Lin GQ, Zhao Q. Synlett, 2018, 29: 1223–1228
Claraz A, Serpier F, Darses S. ACS Catal, 2017, 7: 3410–3413
Partridge BM, Solana González J, Lam HW. Angew Chem Int Ed, 2014, 53: 6523–6527
Shintani R, Isobe S, Takeda M, Hayashi T. Angew Chem Int Ed, 2010, 49: 3795–3798
Miura T, Sasaki T, Nakazawa H, Murakami M. J Am Chem Soc, 2005, 127: 1390–1391
Chen S, Liu ZS, Yang T, Hua Y, Zhou Z, Cheng HG, Zhou Q. Angew Chem Int Ed, 2018, 57: 7161–7165
Shi G, Shao C, Ma X, Gu Y, Zhang Y. ACS Catal, 2018, 8: 3775–3779
Chen S, Wang P, Cheng HG, Yang C, Zhou Q. Chem Sci, 2019, 10: 8384–8389
Wang P, Chen S, Zhou Z, Cheng HG, Zhou Q. Org Lett, 2019, 21: 3323–3327
Mills LR, Graham JM, Patel P, Rousseaux SAL. J Am Chem Soc, 2019, 141: 19257–19262
Malapit CA, Reeves JT, Busacca CA, Howell AR, Senanayake CH. Angew Chem Int Ed, 2016, 55: 326–330
Reeves JT, Malapit CA, Buono FG, Sidhu KP, Marsini MA, Sader CA, Fandrick KR, Busacca CA, Senanayake CH. J Am Chem Soc, 2015, 137: 9481–9488
Chen ZH, Sun RZ, Yao F, Hu XD, Xiang LX, Cong H, Liu WB. J Am Chem Soc, 2022, 144: 4776–4782
Hu XD, Chen ZH, Zhao J, Sun RZ, Zhang H, Qi X, Liu WB. J Am Chem Soc, 2021, 143: 3734–3740
Lu Z, Hu XD, Zhang H, Zhang XW, Cai J, Usman M, Cong H, Liu WB. J Am Chem Soc, 2020, 142: 7328–7333
Malapit CA, Caldwell DR, Luvaga IK, Reeves JT, Volchkov I, Gonnella NC, Han ZS, Busacca CA, Howell AR, Senanayake CH. Angew Chem Int Ed, 2017, 56: 6999–7002
Zhang X, Xie X, Liu Y. Chem Sci, 2016, 7: 5815–5820
Yang J, Chen X, Wang Z. Tetrahedron Lett, 2015, 56: 5673–5675
Korenaga T, Ko A, Uotani K, Tanaka Y, Sakai T. Angew Chem Int Ed, 2011, 50: 10703–10707
Ueura K, Miyamura S, Satoh T, Miura M. J Organomet Chem, 2006, 691: 2821–2826
Lautens M, Roy A, Fukuoka K, Fagnou K, Martín-Matute B. J Am Chem Soc, 2001, 123: 5358–5359
Sasabe H, Kido J. Chem Mater, 2010, 23: 621–630
Ueura K, Satoh T, Miura M. Org Lett, 2005, 7: 2229–2231
Li H, Zhang S, Yu X, Feng X, Yamamoto Y, Bao M. Chem Commun, 2019, 55: 1209–1212
Choi K, Park H, Lee C. J Am Chem Soc, 2018, 140: 10407–10411
He ZT, Tian B, Fukui Y, Tong X, Tian P, Lin GQ. Angew Chem Int Ed, 2013, 52: 5314–5318
Miura T, Shimada M, Murakami M. J Am Chem Soc, 2005, 127: 1094–1095
Shintani R, Tsurusaki A, Okamoto K, Hayashi T. Angew Chem Int Ed, 2005, 44: 3909–3912
Miura T, Shimada M, Murakami M. Angew Chem Int Ed, 2005, 44: 7598–7600
Fang X, Yu P, Prina Cerai G, Morandi B. Chem Eur J, 2016, 22: 15629–15633
Fang X, Yu P, Morandi B. Science, 2016, 351: 832–836
Chen C, Hou C, Chen P, Liu G. Chin J Chem, 2020, 38: 346–350
Aikawa K, Maruyama K, Honda K, Mikami K. Org Lett, 2015, 17: 4882–4885
Katritzky AR, Pilarski B, Urogdi L. Synthesis, 1989, 1989(12): 949–950
Lu X, Huang Y. Org Chem Front, 2021, 8: 3008–3013
Zhang SS, Hu TJ, Li MY, Song YK, Yang XD, Feng CG, Lin GQ. Angew Chem Int Ed, 2019, 58: 3387–3391
Liu N, Yao J, Yin L, Lu T, Tian Z, Dou X. ACS Catal, 2019, 9: 6857–6863
Ming J, Shi Q, Hayashi T. Chem Sci, 2018, 9: 7700–7704
Partridge BM, Callingham M, Lewis W, Lam HW. Angew Chem Int Ed, 2017, 56: 7227–7232
Callingham M, Partridge BM, Lewis W, Lam HW. Angew Chem Int Ed, 2017, 56: 16352–16356
Ma S, Gu Z. Angew Chem Int Ed, 2005, 44: 7512–7517
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21971074), the Natural Science Foundation of Guangdong Province (2022A1515010660, 2021A1515220024), and Natural Science Foundation of GuangZhou (202102020982). We further thank Dr. H. Jiang for technical assiatance with IR, Prof. Daniel J. Weix for the discussion and helpful suggestions about this paper. Dr. Y. Zhao from Francool Technology (Shenzhen) Co. Ltd. is gratefully acknowledged for his help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare no conflict of interest.
Additional information
Supporting information
The Supporting Information is available online at http://chem.scichina.com and http://link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information
11426_2023_1645_MOESM1_ESM.pdf
Redox-neutral ipso/ortho alkenylcyanation of (hetero)arylboronic acid enabled by 1,4-rhodium migration and fragmenta-tion
Rights and permissions
About this article
Cite this article
Guo, C., Xing, D., Jiang, H. et al. Redox-neutral ipso/ortho alkenylcyanation of (hetero)arylboronic acid enabled by 1,4-rhodium migration and fragmentation. Sci. China Chem. 66, 2283–2291 (2023). https://doi.org/10.1007/s11426-023-1645-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-023-1645-x