Abstract
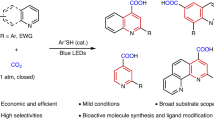
Difunctionalizing carboxylation of alkynes with CO2 is a sustainable and important strategy to generate valuable acrylate derivatives from both readily available starting materials. Such protocols, however, always suffer from the use of excess metallic reagents and transition metal residue. Herein, we report the first thio-carboxylation of alkynes with thiophenols and CO2, which is a visible-light-driven and transition metal-free process. In contrast to previous carboxylations of alkynes via two-electron activation of CO2, mechanistic and computational investigations suggest that the single-electron activation of CO2 is involved in the thio-carboxylation, rendering unique β-carboxylation. The following cyclizing acylation affords important thiochromones efficiently. Moreover, the one-pot method features mild reaction conditions (room temperature, 1 atmosphere of CO2), high chemo- and regio-selectivity, easy scalability and facile derivatization of products to bioactive compounds.

Similar content being viewed by others
References
Liu Q, Wu L, Jackstell R, Beller M. Nat Commun, 2015, 6: 5933
Song QW, Zhou ZH, He LN. Green Chem, 2017, 19: 3707–3728
Grignard B, Gennen S, Jérôme C, Kleij AW, Detrembleur C. Chem Soc Rev, 2019, 48: 4466–4514
Burkart MD, Hazari N, Tway CL, Zeitler EL. ACS Catal, 2019, 9: 7937–7956
He M, Sun Y, Han B. Angew Chem Int Ed, 2022, 61: e202112835
Wang S, Xi C. Chem Soc Rev, 2019, 48: 382–404
Song L, Jiang YX, Zhang Z, Gui YY, Zhou XY, Yu DG. Chem Commun, 2020, 56: 8355–8367
Tortajada A, JuliáHernández F, Börjesson M, Moragas T, Martin R. Angew Chem Int Ed, 2018, 57: 15948–15982
Zhang Z, Ye JH, Ju T, Liao LL, Huang H, Gui YY, Zhou WJ, Yu DG. ACS Catal, 2020, 10: 10871–10885
Zhang L, Li Z, Takimoto M, Hou Z. Chem Rec, 2020, 20: 494–512
Zhang Z, Ye JH, Wu DS, Zhou YQ, Yu DG. Chem Asian J, 2018, 13: 2292–2306
Zhang L, Hou Z. Curr Opin Green Sustain Chem, 2017, 3: 17–21
Wittcoff HA, Reuben BG., Plotkin JS. Industrial Organic Chemicals. 3rd ed. Hoboken: John Wiley & Sons, 2013
Fujihara T, Xu T, Semba K, Terao J, Tsuji Y. Angew Chem Int Ed, 2011, 50: 523–527
Li S, Yuan W, Ma S. Angew Chem Int Ed, 2011, 50: 2578–2582
Wang X, Nakajima M, Martin R. J Am Chem Soc, 2015, 137: 8924–8927
Shao P, Wang S, Du G, Xi C. RSC Adv, 2017, 7: 3534–3539
Xiong W, Shi F, Cheng R, Zhu B, Wang L, Chen P, Lou H, Wu W, Qi C, Lei M, Jiang H. ACS Catal, 2020, 10: 7968–7978
Wang MM, Lu SM, Paridala K, Li C. Chem Commun, 2021, 57: 1230–1233
Shimizu K, Takimoto M, Sato Y, Mori M. Org Lett, 2005, 7: 195–197
Fujihara T, Tani Y, Semba K, Terao J, Tsuji Y. Angew Chem Int Ed, 2012, 51: 11487–11490
Li S, Ma S. Adv Synth Catal, 2012, 354: 2387–2394
Zhang L, Cheng J, Carry B, Hou Z. J Am Chem Soc, 2012, 134: 14314–14317
Wang X, Liu Y, Martin R. J Am Chem Soc, 2015, 137: 6476–6479
Nogi K, Fujihara T, Terao J, Tsuji Y. J Am Chem Soc, 2016, 138: 5547–5550
Stephenson CRJ, Yoon TP, MacMillan DWC. Visible Light Photocatalysis in Organic Chemistry. Hoboken: Wiley-VCH, 2018
Liu Q, Wu LZ. Natl Sci Rev, 2017, 4: 359–380
Marzo L, Pagire SK, Reiser O, König B. Angew Chem Int Ed, 2018, 57: 10034–10072
Chen Y, Lu LQ, Yu DG, Zhu CJ, Xiao WJ. Sci China Chem, 2019, 62: 24–57
Buzzetti L, Crisenza GEM, Melchiorre P. Angew Chem Int Ed, 2019, 58: 3730–3747
Sumida Y, Ohmiya H. Chem Soc Rev, 2021, 50: 6320–6332
Yeung CS. Angew Chem Int Ed, 2019, 58: 5492–5502
Fan Z, Zhang Z, Xi C. ChemSusChem, 2020, 13: 6201–6218
He X, Qiu LQ, Wang WJ, Chen KH, He LN. Green Chem, 2020, 22: 7301–7320
Cai B, Cheo HW, Liu T, Wu J. Angew Chem Int Ed, 2021, 60: 18950–18980
Ye JH, Ju T, Huang H, Liao LL, Yu DG. Acc Chem Res, 2021, 54: 2518–2531
Sahoo B, Bellotti P, Juliá-Hernández F, Meng Q, Crespi S, König B, Martin R. Chem Eur J, 2019, 25: 9001–9005
Meng QY, Schirmer TE, Berger AL, Donabauer K, König B. J Am Chem Soc, 2019, 141: 11393–11397
Song L, Fu D, Chen L, Jiang Y, Ye J, Zhu L, Lan Y, Fu Q, Yu D. Angew Chem Int Ed, 2020, 59: 21121–21128
Schmalzbauer M, Svejstrup TD, Fricke F, Brandt P, Johansson MJ, Bergonzini G, König B. Chem, 2020, 6: 2658–2672
Meng QY, Wang S, König B. Angew Chem Int Ed, 2017, 56: 13426–13430
Shimomaki K, Murata K, Martin R, Iwasawa N. J Am Chem Soc, 2017, 139: 9467–9470
Liao LL, Cao GM, Ye JH, Sun GQ, Zhou WJ, Gui YY, Yan SS, Shen G, Yu DG. J Am Chem Soc, 2018, 140: 17338–17342
Zhu C, Zhang YF, Liu ZY, Zhou L, Liu H, Feng C. Chem Sci, 2019, 10: 6721–6726
Yan SS, Liu SH, Chen L, Bo ZY, Jing K, Gao TY, Yu B, Lan Y, Luo SP, Yu DG. Chem, 2021, 7: 3099–3113
Ran CK, Niu YN, Song L, Wei MK, Cao YF, Luo SP, Yu YM, Liao LL, Yu DG. ACS Catal, 2022, 12: 18–24
Murata K, Numasawa N, Shimomaki K, Takaya J, Iwasawa N. Chem Commun, 2017, 53: 3098–3101
Yatham VR, Shen Y, Martin R. Angew Chem Int Ed, 2017, 56: 10915–10919
Ye JH, Miao M, Huang H, Yan SS, Yin ZB, Zhou WJ, Yu DG. Angew Chem Int Ed, 2017, 56: 15416–15420
Ju T, Fu Q, Ye J, Zhang Z, Liao L, Yan S, Tian X, Luo S, Li J, Yu D. Angew Chem Int Ed, 2018, 57: 13897–13901
Hou J, Ee A, Cao H, Ong H, Xu J, Wu J. Angew Chem Int Ed, 2018, 57: 17220–17224
Hou J, Ee A, Feng W, Xu JH, Zhao Y, Wu J. J Am Chem Soc, 2018, 140: 5257–5263
Fan X, Gong X, Ma M, Wang R, Walsh PJ. Nat Commun, 2018, 9: 4936
Meng QY, Wang S, Huff GS, König B. J Am Chem Soc, 2018, 140: 3198–3201
Fu Q, Bo ZY, Ye JH, Ju T, Huang H, Liao LL, Yu DG. Nat Commun, 2019, 10: 3592
Zhou WJ, Wang ZH, Liao LL, Jiang YX, Cao KG, Ju T, Li Y, Cao GM, Yu DG. Nat Commun, 2020, 11: 3263
Wang H, Gao Y, Zhou C, Li G. J Am Chem Soc, 2020, 142: 8122–8129
Huang H, Ye JH, Zhu L, Ran CK, Miao M, Wang W, Chen H, Zhou WJ, Lan Y, Yu B, Yu DG. CCS Chem, 2021, 3: 1746–1756
Liao LL, Cao GM, Jiang YX, Jin XH, Hu XL, Chruma JJ, Sun GQ, Gui YY, Yu DG. J Am Chem Soc, 2021, 143: 2812–2821
Ju T, Zhou YQ, Cao KG, Fu Q, Ye JH, Sun GQ, Liu XF, Chen L, Liao LL, Yu DG. Nat Catal, 2021, 4: 304–311
Cao GM, Hu XL, Liao LL, Yan SS, Song L, Chruma JJ, Gong L, Yu DG. Nat Commun, 2021, 12: 3306
Razdan RK, Bruni RJ, Mehta AC, Weinhardt KK, Papanastassiou ZB. J Med Chem, 1978, 21: 643–649
Dhanak D, Keenan RM, Burton G, Kaura A, Darcy MG, Shah DH, Ridgers LH, Breen A, Lavery P, Tew DG, West A. Bioorg Med Chem Lett, 1998, 8: 3677–3682
Dong J, Zhang Q, Meng Q, Wang Z, Li S, Cui J. Mini-Rev Med Chem, 2018, 18: 1714–1732
Hamama WS, Sofan MA, EL-Hawary II, Zoorob HH. Synth Commun, 2021, 51: 514–540
Manjolinho F, Arndt M, Gooßen K, Gooßen LJ. ACS Catal, 2012, 2: 2014–2021
Zhou C, Dubrovsky AV, Larock RC. J Org Chem, 2006, 71: 1626–1632
Nakazumi H, Ueyama T, Sonoda H, Kitao T. Bull Chem Soc Jpn, 1984, 57: 2323–2324
Nakazumi H, Ueyama T, Endo T, Kitao T. Bull Chem Soc Jpn, 1983, 56: 1251–1252
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22225106, 21822108, 21822303), the Sichuan Science and Technology Program (20CXTD0112), the Central Government Funds of Guiding Local Scientific and Technological Development for Sichuan Province (2021ZYD0063), the Fundamental Research Funds from Sichuan University (2020SCUNL102) and the Fundamental Research Funds for the Central Universities. We also thank Xiaoyan Wang from the Analysis and Testing Center of Sichuan University as well as Jing Li, Qin-Fang Zhang and Dongyan Deng from the College of Chemistry at Sichuan University for compound testing.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare the following competing financial interest(s): A Chinese Patent on this work has been applied with the number 202210655518.3.
Supporting information
The supporting information is available online at chem.scichina.com and link.springer.com/journal/11426. The supporting materials are published as submitted, without typesetting or editing. The responsibility for scientific accuracy and content remains entirely with the authors.
Supporting Information for
Rights and permissions
About this article
Cite this article
Miao, M., Zhu, L., Zhao, H. et al. Visible-light-driven thio-carboxylation of alkynes with CO2: facile synthesis of thiochromones. Sci. China Chem. 66, 1457–1466 (2023). https://doi.org/10.1007/s11426-022-1554-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-022-1554-x