Abstract
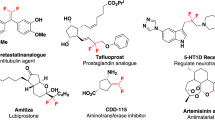
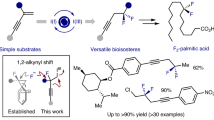
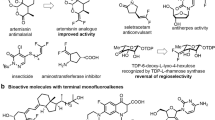
β-Difluorinated alkyl halides are of significant value in the modular synthesis of gem-difluorinated molecules. An exclusive 1,2-halo migratory gem-difluorination of vinyl halides with in situ-generated PhIF2·HF is described. This protocol provides a general and practical approach towards a wide variety of β-difluorinated alkyl bromides. Both α- and β-bromoalkyl alkenes are suitable substrates, leading to two distinct types of products. The extension of this protocol to vinyl chloride and iodide are also feasible. The synthetic versatility of this method has been highlighted by the late-stage modification of complex small molecules and further transformations of the β-difluorinated alkyl halides to valuable CF2-containing compounds.

Similar content being viewed by others
References
For selected reviews, see: (a) Jeschke P. ChemBioChem, 2004, 5: 570–589
Shah P, Westwell AD. J Enzyme Inhibit Med Chem, 2007, 22: 527–540
Hagmann WK. J Med Chem, 2008, 51: 4359–4369
Purser S, Moore PR, Swallow S, Gouverneur V. Chem Soc Rev, 2008, 37: 320–330
(e) Meanwell, NA. J Med Chem, 2011, 54, 2529–2591
Liang T, Neumann CN, Ritter T. Angew Chem Int Ed, 2013, 52: 8214–8264
Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. J Med Chem, 2015, 58: 8315–8359
Yerien DE, Bonesi S, Postigo A. Org Biomol Chem, 2016, 14: 8398–8427
Mei H, Han J, Klika KD, Izawa K, Sato T, Meanwell NA, Soloshonok VA. Eur J Med Chem, 2020, 186: 111826
Dubowchik GM, Vrudhula VM, Dasgupta B, Ditta J, Chen T, Sheriff S, Sipman K, Witmer M, Tredup J, Vyas DM, Verdoorn TA, Bollini S, Vinitsky A. Org Lett, 2001, 3: 3987–3990
Ye XM, Konradi AW, Smith J, Aubele DL, Garofalo AW, Marugg J, Neitzel ML, Semko CM, Sham HL, Sun M, Truong AP, Wu J, Zhang H, Goldbach E, Sauer JM, Brigham EF, Bova M, Basi GS. Bioorg Med Chem Lett, 2010, 20: 3502–3506
Zhou Q, Ruffoni A, Gianatassio R, Fujiwara Y, Sella E, Shabat D, Baran PS. Angew Chem Int Ed, 2013, 52: 3949–3952
Meanwell NA. J Med Chem, 2018, 61: 5822–5880
Kitas EA, Galley G, Jakob-Roetne R, Flohr A, Wostl W, Mauser H, Alker AM, Czech C, Ozmen L, David-Pierson P, Reinhardt D, Jacobsen H. Bioorg Med Chem Lett, 2008, 18: 304–308
Bégué JP, Bonnet-Delpon D. J Fluorine Chem, 2006, 127: 992–1012
Wang J, Sánchez-Roselló M, Aceña JL, del Pozo C, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H. Chem Rev, 2014, 114: 2432–2506
Erickson JA, McLoughlin JI. J Org Chem, 1995, 60: 1626–1631
Xu Y, Qian L, Pontsler AV, McIntyre TM, Prestwich GD. Tetrahedron, 2004, 60: 43–49
Martínez MD, Luna L, Tesio AY, Feresin GE, Durán FJ, Burton G. J Pharmacy Pharmacol, 2016, 68: 233–244
Sessler CD, Rahm M, Becker S, Goldberg JM, Wang F, Lippard SJ. J Am Chem Soc, 2017, 139: 9325–9332
Zafrani Y, Yeffet D, Sod-Moriah G, Berliner A, Amir D, Marciano D, Gershonov E, Saphier S. J Med Chem, 2017, 60: 797–804
Zafrani Y, Sod-Moriah G, Yeffet D, Berliner A, Amir D, Marciano D, Elias S, Katalan S, Ashkenazi N, Madmon M, Gershonov E, Saphier S. J Med Chem, 2019, 62: 5628–5637
For selected reviews, see: (a) Pan X, Xia H, Wu J. Org Chem Front, 2016, 3: 1163–1185
Yerien DE, Barata-Vallejo S, Postigo A. Chem Eur J, 2017, 23: 14676–14701
Feng Z, Xiao YL, Zhang X. Acc Chem Res, 2018, 51: 2264–2278
Hu XS, Yu JS, Zhou J. Chem Commun, 2019, 55: 13638–13648
Dong DQ, Yang H, Shi JL, Si WJ, Wang ZL, Xu XM. Org Chem Front, 2020, 7: 2538–2575; For selected recent examples of copper-catalyzed difluoromethylation, see
Zeng X, Yan W, Zacate SB, Chao TH, Sun X, Cao Z, Bradford KGE, Paeth M, Tyndall SB, Yang K, Kuo TC, Cheng MJ, Liu W. J Am Chem Soc, 2019, 141: 11398–11403
Zeng X, Yan W, Paeth M, Zacate SB, Hong PH, Wang Y, Yang D, Yang K, Yan T, Song C, Cao Z, Cheng MJ, Liu W. J Am Chem Soc, 2019, 141: 19941–19949
Hara S, Nakahigashi J, Ishi-i K, Fukuhara T, Yoneda N. Tetrahedron Lett, 1998, 39: 2589–2592
For examples of 1,2-difluorination of alkenes, see: (a) Molnár IG, Gilmour R. J Am Chem Soc, 2016, 138: 5004–5007
Banik SM, Medley JW, Jacobsen EN. J Am Chem Soc, 2016, 138: 5000–5003
Scheidt F, Schäfer M, Sarie JC, Daniliuc CG, Molloy JJ, Gilmour R. Angew Chem Int Ed, 2018, 57: 16431–16435
Haj MK, Banik SM, Jacobsen EN. Org Lett, 2019, 21: 4919–4923
Doobary S, Sedikides AT, Caldora HP, Poole DL, Lennox AJJ. Angew Chem Int Ed, 2020, 59: 1155–1160
For examples of 1,1-difluorination of alkenes, see: (a) Ilchenko NO, Tasch BOA, Szabó KJ. Angew Chem Int Ed, 2014, 53: 12897–12901
Kitamura T, Muta K, Oyamada J. J Org Chem, 2015, 80: 10431–10436
Banik SM, Medley JW, Jacobsen EN. Science, 2016, 353: 51–54
Ilchenko NO, Szabó KJ. J Fluorine Chem, 2017, 203: 104–109
Zhao Z, Racicot L, Murphy GK. Angew Chem Int Ed, 2017, 56: 11620–11623
Kitamura T, Yoshida K, Mizuno S, Miyake A, Oyamada J. J Org Chem, 2018, 83: 14853–14860
Scheidt F, Neufeld J, Schäfer M, Thiehoff C, Gilmour R. Org Lett, 2018, 20: 8073–8076
Zhao Z, To AJ, Murphy GK. Chem Commun, 2019, 55: 14821–14824
For reviews on iodine(III)-mediated halogenations, see: (a) Kohlhepp SV, Gulder T. Chem Soc Rev, 2016, 45: 6270–6288
Arnold AM, Ulmer A, Gulder T. Chem Eur J, 2016, 22: 8728–8739
Li H, Reddy BRP, Bi X. Org Lett, 2019, 21: 9358–9362
Ning Y, Sivaguru P, Zanoni G, Anderson EA, Bi X. Chem, 2020, 6: 486–496
For selected recent examples on the introduction of the CF2 moiety into complex molecules using a CF2-X bond, see: (a) Yu YB, He GZ, Zhang X. Angew Chem Int Ed, 2014, 53: 10457–10461
Nie X, Cheng C, Zhu G. Angew Chem Int Ed, 2017, 56: 1898–1902
Xiang H, Zhao QL, Xia PJ, Xiao JA, Ye ZP, Xie X, Sheng H, Chen XQ, Yang H. Org Lett, 2018, 20: 1363–1366
Wang H, Jui NT. J Am Chem Soc, 2018, 140: 163–166
Zhu E, Liu XX, Wang AJ, Mao T, Zhao L, Zhang X, He CY. Chem Commun, 2019, 55: 12259–12262
Li L, Luo H, Zhao Z, Li Y, Zhou Q, Xu J, Li J, Ma YN. Org Lett, 2019, 21: 9228–9231
Tu HY, Wang F, Huo L, Li Y, Zhu S, Zhao X, Li H, Qing FL, Chu L. J Am Chem Soc, 2020, jacs.0c03708
Lv WX, Li Q, Li JL, Li Z, Lin E, Tan DH, Cai YH, Fan WX, Wang H. Angew Chem Int Ed, 2018, 57: 16544–16548
Levin MD, Ovian JM, Read JA, Sigman MS, Jacobsen EN. J Am Chem Soc, 2020, 142: 14831–14837
CCDC-2031317 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre viahttp://www.ccdc.cam.ac.uk/data_request/cif
Sun X, Li X, Song S, Zhu Y, Liang YF, Jiao N. J Am Chem Soc, 2015, 137: 6059–6066
Zhou B, Yan T, Xue XS, Cheng JP. Org Lett, 2016, 18: 6128–6131
Zhou B, Haj MK, Jacobsen EN, Houk KN, Xue XS. J Am Chem Soc, 2018, 140: 15206–15218
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21961047, 21901266, 21971261, 22022114).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, C., Liao, Y., Tan, X. et al. Hypervalent iodine-mediated gem-difluorination of vinyl halides enabled by exclusive 1,2-halo migration. Sci. China Chem. 64, 999–1003 (2021). https://doi.org/10.1007/s11426-021-9965-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-9965-9