Abstract
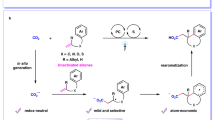
Herein, we report a novel protocol for visible-light-driven alkylative carboxylation of alkenes with CO2 in the absence of external photocatalyst. Under the irradiation of visible light, a variety of 4-alkyl-1,4-dihydropyridines (alkyl-DHPs) serve as not only alkyl radical precursors but also photoexcited reductants probably with the potential to reduce benzyl radicals. Several styrenes and acrylates are applicable in this reaction to give structurally diverse carboxylic acids in good to excellent yields. These reactions feature mild reaction conditions (1 atm of CO2, room temperature, visible light, photocatalyst- and transition metal-free), good functional group tolerance, easy scalability, as well as high regio-, and chemo-selectivity. Mechanistic investigations provide evidence that alkyl radical, benzyl radical and carbanion might be involved in this reaction, providing a novel strategy for CO2 utilization.

Similar content being viewed by others
References
Das S. CO2as a Building Block in Organic Synthesis. Weinheim: Wiley-VCH, 2020
Liu Q, Wu L, Jackstell R, Beller M. Nat Commun, 2015, 6: 5933
Song QW, Zhou ZH, He LN. Green Chem, 2017, 19: 3707–3728
Wang S, Xi C. Chem Soc Rev, 2019, 48: 382–404
Grignard B, Gennen S, Jérôme C, Kleij AW, Detrembleur C. Chem Soc Rev, 2019, 48: 4466–4514
Maag H. Prodrugs of Carboxylic Acids. New York: Springer, 2007
Ye JH, Ju T, Huang H, Liao LL, Yu DG. Acc Chem Res, 2021, 54: 2518–2531
Zhang L, Hou Z. Curr Opin Green Sustain Chem, 2017, 3: 17–21
Tortajada A, Juliá-Hernández F, Börjesson M, Moragas T, Martin R. Angew Chem Int Ed, 2018, 57: 15948–15982
Yan SS, Fu Q, Liao LL, Sun GQ, Ye JH, Gong L, Bo-Xue YZ, Yu DG. Coord Chem Rev, 2018, 374: 439–463
Luan YX, Ye M. Tetrahedron Lett, 2018, 59: 853–861
Yan M, Kawamata Y, Baran PS. Chem Rev, 2017, 117: 13230–13319
Cao Y, He X, Wang N, Li HR, He LN. Chin J Chem, 2018, 36: 644–659
Chen Y, Lu LQ, Yu DG, Zhu CJ, Xiao WJ. Sci China Chem, 2019, 62: 24–57
Zhang Z, Gong L, Zhou XY, Yan SS, Li J, Yu DG. Acta Chim Sin, 2019, 77: 783–793
Hou J, Li JS, Wu J. Asian J Org Chem, 2018, 7: 1439–1447
Yeung CS. Angew Chem Int Ed, 2019, 58: 5492–5502
Zhang Z, Ye JH, Ju T, Liao LL, Huang H, Gui YY, Zhou WJ, Yu DG. ACS Catal, 2020, 10: 10871–10885
He X, Qiu LQ, Wang WJ, Chen KH, He LN. Green Chem, 2020, 22: 7301–7320
Prier CK, Rankic DA, MacMillan DWC. Chem Rev, 2013, 113: 5322–5363
Romero NA, Nicewicz DA. Chem Rev, 2016, 116: 10075–10166
Shaw MH, Twilton J, MacMillan DWC. J Org Chem, 2016, 81: 6898–6926
Murata K, Numasawa N, Shimomaki K, Takaya J, Iwasawa N. Chem Commun, 2017, 53: 3098–3101
Murata K, Numasawa N, Shimomaki K, Takaya J, Iwasawa N. Front Chem, 2019, 7: 371
Seo H, Liu A, Jamison TF. J Am Chem Soc, 2017, 139: 13969–13972
Meng QY, Wang S, Huff GS, König B. J Am Chem Soc, 2018, 140: 3198–3201
Huang H, Ye JH, Zhu L, Ran CK, Miao M, Wang W, Chen H, Zhou WJ, Lan Y, Yu B, Yu DG. CCS Chem, 2020, 2: 1746–1756
Yatham VR, Shen Y, Martin R. Angew Chem Int Ed, 2017, 56: 10915–10919
Hou J, Ee A, Cao H, Ong HW, Xu JH, Wu J. Angew Chem Int Ed, 2018, 57: 17220–17224
Zhang B, Yi Y, Wu ZQ, Chen C, Xi C. Green Chem, 2020, 22: 5961–5965
Liao LL, Cao GM, Jiang YX, Jin XH, Hu XL, Chruma JJ, Sun GQ, Gui YY, Yu DG. J Am Chem Soc, 2021, 143: 2812–2821
Fu Q, Bo ZY, Ye JH, Ju T, Huang H, Liao LL, Yu DG. Nat Commun, 2019, 10: 3592
Wang H, Gao Y, Zhou C, Li G. J Am Chem Soc, 2020, 142: 8122–8129
Zhou WJ, Wang ZH, Liao LL, Jiang YX, Cao KG, Ju T, Li Y, Cao GM, Yu DG. Nat Commun, 2020, 11: 3263
During the revision of this manuscript, Sun and Cheng reported an elegant and very similar visible-light photoredox-catalyzed α-aminomethyl carboxylation of styrenes with CO2, see: Zhou C, Li M, Sun J, Cheng J, Sun S. Org Lett, 2021, 23: 2895–2899
With electrochemical methods, Lin reported a reductive carbofunctionalization of alkenes with CO2, see: Zhang W, Lin S. J Am Chem Soc, 2020, 142: 20661–20670
Ju T, Zhou YQ, Cao KG, Fu Q, Ye JH, Sun GQ, Liu XF, Chen L, Liao LL, Yu DG. Nat Catal, 2021, 4: 304–311
Johnson, MK, Smith, AD. Iron-sulfur proteins. In: King RB, Ed. Encyclopedia of Inorganic Chemistry. 2nd Ed. Weinheim: Wiley-VCH, 2005
Rickard D, Luther GW. Chem Rev, 2007, 107: 514–562
Ye JH, Miao M, Huang H, Yan SS, Yin ZB, Zhou WJ, Yu DG. Angew Chem Int Ed, 2017, 56: 15416–15420
Zheng C, You SL. Chem Soc Rev, 2012, 41: 2498–2518
Jung J, Kim J, Park G, You Y, Cho EJ. Adv Synth Catal, 2016, 358: 74–80
Panferova LI, Tsymbal AV, Levin VV, Struchkova MI, Dilman AD. Org Lett, 2016, 18: 996–999
Chen W, Tao H, Huang W, Wang G, Li S, Cheng X, Li G. Chem Eur J, 2016, 22: 9546–9550
Wang PZ, Chen JR, Xiao WJ. Org Biomol Chem, 2019, 17: 6936–6951
Buzzetti L, Prieto A, Roy SR, Melchiorre P. Angew Chem Int Ed, 2017, 56: 15039–15043
Goti G, Bieszczad B, Vega-Peñaloza A, Melchiorre P. Angew Chem Int Ed, 2019, 58: 1213–1217
van Leeuwen T, Buzzetti L, Perego LA, Melchiorre P. Angew Chem Int Ed, 2019, 58: 4953–4957
Bieszczad B, Perego LA, Melchiorre P. Angew Chem Int Ed, 2019, 58: 16878–16883
Pope BM, Yamamoto Y, Tarbell DS. Org Synth, 1977, 57: 45
Dean CS, Tarbell DS, Friederang AW. J Org Chem, 1970, 35: 3393–3397
Marzo L, Pagire SK, Reiser O, König B. Angew Chem Int Ed, 2018, 57: 10034–10072
Liu B, Lim CH, Miyake GM. J Am Chem Soc, 2017, 139: 13616–13619
Yang M, Cao T, Xu T, Liao S. Org Lett, 2019, 21: 8673–8678
Li G, Yan Q, Gan Z, Li Q, Dou X, Yang D. Org Lett, 2019, 21: 7938–7942
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21822108, 21772129), the Fok Ying Tung Education Foundation (161013), Sichuan Science and Technology Program (20CXTD0112), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Niu, YN., Jin, XH., Liao, LL. et al. Visible-light-driven external-photocatalyst-free alkylative carboxylation of alkenes with CO2. Sci. China Chem. 64, 1164–1169 (2021). https://doi.org/10.1007/s11426-021-1004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-021-1004-y