Abstract
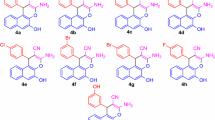
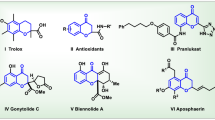
Chromenes represent an important class of six-membered heterocycles and have drawn tremendous attention in recent years. In this article, we report a convenient and practical synthesis of this heterocycle by a silver (I)-catalyzed cycloaddition reaction between in situ generated ortho-quinone methides and N-allenamides. Diverse 4H-chromenes were synthesized in good to excellent yields under very mild conditions.
Similar content being viewed by others
References
Jiang ZZ, Gao A, Li H, Chen D, Ding CH, Xu B, Hou XL. Chem Asian J, 2017, 12: 3119–3122
Graham TJA, Doyle AG. Org Lett, 2012, 14: 1616–1619
Shestopalov AM, Litvinov YM, Rodinovskaya LA, Malyshev OR, Semenova MN, Semenov VV. ACS Comb Sci, 2012, 14: 484–490
Ren Q, Siau WY, Du Z, Zhang K, Wang J. Chem Eur J, 2011, 17: 7781–7785
Malakar CC, Schmidt D, Conrad J, Beifuss U. Org Lett, 2011, 13: 1972–1975
Hufford CD, Oguntimein BO, Baker JK. J Org Chem, 1981, 46: 3073–3078
Chansakaow S, Ishikawa T, Seki H, Sekine K, Okada M, Chaichantipyuth C. J Nat Prod, 2000, 63: 173–175
Das SG, Doshi JM, Tian D, Addo SN, Srinivasan B, Hermanson DL, Xing C. J Med Chem, 2009, 52: 5937–5949
Li M, Zhang B, Gu Y. Green Chem, 2012, 14: 2421–2428
Liu Y, Qian J, Lou S, Zhu J, Xu Z. J Org Chem, 2010, 75: 1309–1312
Singh SN, Bopanni R, Jayaprakash S, Reddy KV, Ashfaq MA, Kumar KS, Pal M. RSC Adv, 2014, 4: 24870–24873
Bai WJ, David JG, Feng ZG, Weaver MG, Wu KL, Pettus TRR. Acc Chem Res, 2014, 47: 3655–3664
van de Water RW, Pettus TRR. Tetrahedron, 2002, 58: 5367–5405
Alden-Danforth E, Scerba MT, Lectka T. Org Lett, 2008, 10: 4951–4953
Hsiao CC, Liao HH, Rueping M. Angew Chem Int Ed, 2014, 53: 13258–13263
Pathak TP, Sigman MS. J Org Chem, 2011, 76: 9210–9215
Caruana L, Fochi M, Bernardi L. Molecules, 2015, 20: 11733–11764
Wang Z, Sun J. Synthesis, 2015, 47: 3629–3644
Ai W, Liao D, Lei X. Chin J Org Chem, 2015, 35: 1615–1626
Jana R, Pathak TP, Jensen KH, Sigman MS. Org Lett, 2012, 14: 4074–4077
Izquierdo J, Orue A, Scheidt KA. J Am Chem Soc, 2013, 135: 10634–10637
Lv H, Jia WQ, Sun LH, Ye S. Angew Chem Int Ed, 2013, 52: 8607–8610
Huang Y, Hayashi T. J Am Chem Soc, 2015, 137: 7556–7559
Zhao W, Wang Z, Chu B, Sun J. Angew Chem Int Ed, 2015, 54: 1910–1913
Saha S, Schneider C. Org Lett, 2015, 17: 648–651
Tsui GC, Liu L, List B. Angew Chem Int Ed, 2015, 54: 7703–7706
Zhao JJ, Sun SB, He SH, Wu Q, Shi F. Angew Chem Int Ed, 2015, 54: 5460–5464
Guo W, Wu B, Zhou X, Chen P, Wang X, Zhou YG, Liu Y, Li C. Angew Chem Int Ed, 2015, 54: 4522–4526
Wang Z, Ai F, Wang Z, Zhao W, Zhu G, Lin Z, Sun J. J Am Chem Soc, 2015, 137: 383–389
Yu XY, Chen JR, Wei Q, Cheng HG, Liu ZC, Xiao WJ. Chem Eur J, 2016, 22: 6774–6778
Osipov DV, Osyanin VA, Khaysanova GD, Masterova ER, Krasnikov PE, Klimochkin YN. J Org Chem, 2018, 83: 4775–4785
El-Sepelgy O, Haseloff S, Alamsetti SK, Schneider C. Angew Chem Int Ed, 2014, 53: 7923–7927
Hsiao CC, Raja S, Liao HH, Atodiresei I, Rueping M. Angew Chem Int Ed, 2015, 54: 5762–5765
Xie Y, List B. Angew Chem Int Ed, 2017, 56: 4936–4940
Wang Z, Sun J. Org Lett, 2016, 19: 2334–2337
Lukashenko AV, Osyanin VA, Osipov DV, Klimochkin YN. J Org Chem, 2017, 82: 1517–1528
Thirupathi N, Tung CH, Xu Z. Adv Synth Catal, 2018, 360: 3585–3589
Faustino H, López F, Castedo L, Mascareñas JL. Chem Sci, 2011, 2: 633–637
Wang Y, Zhang P, Qian D, Zhang J. Angew Chem Int Ed, 2015, 54: 14849–14852
Li XX, Zhu LL, Zhou W, Chen Z. Org Lett, 2012, 14: 436–439
Wang Y, Zhang P, Liu Y, Xia F, Zhang J. Chem Sci, 2015, 6: 5564–5570
Peng S, Cao S, Sun J. Org Lett, 2017, 19: 524–527
Varela I, Faustino H, Díez E, Iglesias-Sigüenza J, Grande-Carmona F, Fernández R, Lassaletta JM, Mascareñas JL, López F. ACS Catal, 2017, 7: 2397–2402
Lu T, Lu Z, Ma ZX, Zhang Y, Hsung RP. Chem Rev, 2013, 113: 4862–4904
Wei LL, Xiong H, Hsung RP. Acc Chem Res, 2003, 36: 773–782
Suárez-Pantiga S, Hernández-Díaz C, Rubio E, González JM. Angew Chem Int Ed, 2012, 51: 11552–11555
Suárez-Pantiga S, Hernández-Díaz C, Piedrafita M, Rubio E, González JM. Adv Synth Catal, 2012, 354: 1651–1657
Sabbatani J, Huang X, Veiros LF, Maulide N. Chem Eur J, 2014, 20: 10636–10639
Liang M, Zhang S, Jia J, Tung CH, Wang J, Xu Z. Org Lett, 2017, 19: 2526–2529
Du JY, Ma YH, Meng FX, Chen BL, Zhang SL, Li QL, Gong SW, Wang DQ, Ma CL. Org Lett, 2018, 20: 4371–4374
Zhang S, Shan C, Zhang S, Yuan L, Wang J, Tung CH, Xing LB, Xu Z. Org Biomol Chem, 2016, 14: 10973–10980
Wang X, Yao Z, Dong S, Wei F, Wang H, Xu Z. Org Lett, 2013, 15: 2234–2237
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21572118, 21750110444), the Natural Science Foundation of Shandong Province (ZR2018MB010), subject construction funds (104.205.2.5) and Tang scholar award of Shandong University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kong, L., Thirupathi, N., Jia, J. et al. Synthesis of 4H-chromenes by silver (I)-catalyzed cycloaddition of ortho-quinone methides with N-allenamides. Sci. China Chem. 62, 80–86 (2019). https://doi.org/10.1007/s11426-018-9359-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-018-9359-4