Abstract
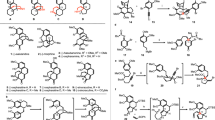
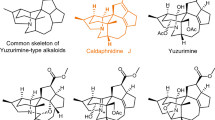
Lycopodium alkaloids, a unique family of biologically important natural products isolated and characterized from various species of Lycopodium (sensu lato), have attracted extensive attention from chemists and pharmacists in the past three decades. Michael addition-based cyclization has been successfully employed as an elegant and efficient ring-construction protocol of constructing key cyclohexanone intermediates in the total synthesis of Lycopodium alkaloids. This mini-review chooses and summarizes several representative total syntheses of various Lycopodium alkaloids in which intramolecular Michael addition severed as the key methodology.
Similar content being viewed by others
References
Bödeker K. Justus Liebigs Ann Chem, 1881, 208: 363–367
Murphy RA, Sarpong R. Chem Eur J, 2014, 20: 42–56
Ferreira A, Rodrigues M, Fortuna A, Falcão A, Alves G. Phytochem Rev, 2016, 15: 51–85
Zhang DB, Chen JJ, Zhang L, Song QY, Gao K. Phytochem Lett, 2014, 10: 76–79
Banejee J, Biswas S, Madhu NR, Karmakar SR, Surjyo Y. J Pharm Phytochem, 2014, 3: 207–210
Morita H, Hirasawa Y, Kobayashi J. Heterocycles, 2009, 77: 679–729
Liu J, Yu C, Zhou Y, Han Y, Qi B, Zhu Y. Acta Chim Sin, 1986, 44: 1035–1040
Liu JS, Zhu YL, Yu CM, Zhou YZ, Han YY, Wu FW, Qi BF. J Chem, 1986, 64: 837–839
Cheng YS, Lu CZ, Ying ZL, Ni WY, Zhang CL, Sang GW. Chin J New Drug Clin Remedies, 1986, 5: 197–199
Zhang RW, Tang XC, Han YY, Sang GW, Zhang YD, Ma YX, Zhang CL, Yang RM. Acta Pharmacol Sin, 1991, 12: 250–252
Ma X, Gang DR. Nat Prod Rep, 2004, 21: 752–772
Ayer WA, Trifonov LS. The Alkaloids San Diego: Acadimic Press, 1994
Yuan C, Chang CT, Axelrod A, Siegel D. J Am Chem Soc, 2010, 132: 5924–5925
Liau BB, Shair MD. J Am Chem Soc, 2010, 132: 9594–9595
White JD, Li Y, Kim J, Terinek M. J Org Chem, 2015, 80: 11806–11817
Li H, Wang X, Hong B, Lei X. J Org Chem, 2013, 78: 800–821
Yang YR, Lai ZW, Shen L, Huang JZ, Wu XD, Yin JL, Wei K. Org Lett, 2010, 12: 3430–3433
Ding R, Fu JG, Xu GQ, Sun BF, Lin GQ. J Org Chem, 2014, 79: 240–250
Cassayre J, Gagosz F, Zard SZ. Angew Chem Int Ed, 2002, 41: 1783–1785
Chandra A, Pigza JA, Han JS, Mutnick D, Johnston JN. J Am Chem Soc, 2009, 131: 3470–3471
Yang Y, Dai M. Synlett, 2014, 25: 2093–2098
Wang X, Li H, Lei X. Synlett, 2013, 24: 1032–1043
Siengalewicz P, Mulzer J, Rinner U. Alkaloids, 2013, 72: 1–151
Nakayama A, Kitajima M, Takayama H. Synlett, 2012, 23: 2014–2024
Williams BM, Trauner D. Angew Chem Int Ed, 2016, 55: 2191–2194
Yang Y, Haskins CW, Zhang W, Low PL, Dai M. Angew Chem Int Ed, 2014, 53: 3922–3925
Nishimura T, Unni AK, Yokoshima S, Fukuyama T. J Am Chem Soc, 2013, 135: 3243–3247
Newton JN, Fischer DF, Sarpong R. Angew Chem Int Ed, 2013, 52: 1726–1730
Zhang J, Wu J, Hong B, Ai W, Wang X, Li H, Lei X. Nat Commun, 2014, 5: 4614
Rulev AY. RSC Adv, 2014, 4: 26002–26012
Moberg C. Angew Chem Int Ed, 2013, 52: 2160–2162
Brahmachari G, Goutam D. Nat Products, 2009: 782–804
Hack D, Dürr AB, Deckers K, Chauhan P, Seling N, Rübenach L, Mertens L, Raabe G, Schoenebeck F, Enders D. Angew Chem Int Ed, 2016, 55: 1797–1800
Perdriau S, Zijlstra DS, Heeres HJ, de Vries JG, Otten E. Angew Chem Int Ed, 2015, 54: 4236–4240
Farley AJM, Sandford C, Dixon DJ. J Am Chem Soc, 2015, 137: 15992–15995
Li J, Huang R, Xing YK, Qiu G, Tao HY, Wang CJ. J Am Chem Soc, 2015, 137: 10124–10127
Cichowicz NR, Kaplan W, Khomutnyk Y, Bhattarai B, Sun Z, Nagorny P. J Am Chem Soc, 2015, 137: 14341–14348
Yang H, Carter RG. J Org Chem, 2010, 75: 4929–4938
Stork G, Kretchmer RA, Schlessinger RH. J Am Chem Soc, 1968, 90: 1647–1648
Ayer WA, Bowman WR, Joseph TC, Smith P. J Am Chem Soc, 1968, 90: 1648–1650
Kim SW, Bando Y, Horii Z. Tetrahedron Lett, 1978, 19: 2293–2294
Heathcock CH, Kleinman EF, Binkley ES. J Am Chem Soc, 1982, 104: 1054–1068
Schumann D, Müller HJ, Naumann A. Lebigs Ann Chem, 1982, 9: 1700–1705
Kraus GA, Hon Y. Heterocycles, 1987, 377–386
Grieco PA, Dai Y. J Am Chem Soc, 1998, 120: 5128–5129
Padwa A, Brodney MA, Marino JP, Sheehan SM. J Org Chem, 1997, 62: 78–87
Mori M, Hori K, Akashi M, Hori M, Sato Y, Nishida M. Angew Chem Int Ed, 1998, 37: 636–637
Colvin EW, Martin J, Parker W, Paphael RA, Shroot B, Doyle M. J Chem Soc Perkin Trans, 1972: 860–870
Yang H, Carter RG, Zakharov LN. J Am Chem Soc, 2008, 130: 9238–9239
Stork G, Winkler JD, Saccomano NA. Tetrahedron Lett, 1983, 24: 465–468
Evans DA, Scheerer JR. Angew Chem Int Ed, 2005, 44: 6038–6042
Burnell RH, Mootoo BS. Can J Chem, 1961, 39: 1090–1093
Burnell RH. J Chem Soc, 1959: 3091–3093
Heathcock CH, Blumenkopf TA, Smith KM. J Org Chem, 1989, 54: 1548–1562
Jung ME, Chang JJ. Org Lett, 2010, 12: 2962–2965
Fang J, Ren J, Wang Z. Tetrahedron Lett, 2008, 49: 6659–6662
Qu JP, Deng C, Zhou J, Sun XL, Tang Y. J Org Chem, 2009, 74: 7684–7689
Takayama H, Katakawa K, Kitajima M, Yamaguchi K. Heterocycles, 2006, 69:223
Tanaka T, Kogure N, Kitajima M, Takayama H. J Org Chem, 2009, 74: 8675–8680
Bradshaw B, Luque-Corredera C, Bonjoch J. Org Lett, 2013, 15: 326–329
Hayashi Y, Gotoh H, Hayashi T, Shoji M. Angew Chem Int Ed, 2005, 44: 4212–4215
Ding XH, Li X, Liu D, Cui WC, Ju X, Wang S, Yao ZJ. Tetrahedron, 2012, 68: 6240–6248
Ge HM, Zhang LD, Tan RX, Yao ZJ. J Am Chem Soc, 2012, 134: 12323–12325
Zhang LD, Zhou TT, Qi SX, Xi J, Yang XL, Yao ZJ. Chem Asian J, 2014, 9: 2740–2744
Zhang LD, Zhong LR, Xi J, Yang XL, Yao ZJ. J Org Chem, 2016, 81: 1899–1904
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhong, LR., Yao, ZJ. Michael addition-based cyclization strategy in the total synthesis of Lycopodium alkaloids. Sci. China Chem. 59, 1079–1087 (2016). https://doi.org/10.1007/s11426-016-0056-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-016-0056-0